Newsletter 2018 Volume 15 - Issue 3

The 27th International Congress of The Transplantation Society that was held in Madrid on June 30-July 5, 2018 proved to be a great success. The scientific program offered state-of-the-art information ensuring an outstanding quality and the various social events provided many opportunities for people to come together and enjoy the best that Madrid has to offer. I wish to thank everyone who worked so hard over the past two years to make all of this possible.
Preparations are already underway for the next TTS Congress, which will be organized in Seoul on September 12-17, 2020. We are all very excited about this Congress and the Program Committee has already started working on ensuring TTS once again offers an innovative and comprehensive scientific program that provides the latest research developments in the field of transplantation. Working in collaboration with our respected colleagues from the Korean Society for Transplantation, we will ensure that the TTS 2020 Congress is a rewarding and unforgettable experience for all our participants attending from around the world.

In the meantime, there are several other events that are being planned for 2019. We are particularly excited about the first International Transplantation Science Meeting (ITS), that is being organized jointly by TTS, the American Society of Transplantation (AST) and the European Society for Organ Transplantation (ESOT) to take place in the Fall of 2019. I have no doubt that this meeting will maintain the same high standards as the previous TTS Transplantation Sciences Symposia of previous years.
I am also incredibly proud to announce the 1st TTS Regional Meeting to be organized in Istanbul, March 28-29, 2019. The theme for the meeting, “Deceased Donation: Expanding the Donor Pool,” will provide valuable insights into medical, legislative, ethical, cultural, and social hurdles that must be overcome to increase deceased donation rates in the region as well as the world. Although organ transplantation has become the treatment of choice for end stage organ disease, it is important to recognize that organ shortage is the greatest challenge facing the field of organ transplantation today. As such, this pilot meeting will be the first of what we hope will be many more organized in each TTS region over the next few years. In this manner, we hope to reach out to our colleagues in each region and address their individual concerns and the unique challenges that they face.

Finally, as the incoming President of TTS, I wish to express my excitement as we begin this new term. Over the next two years I look forward to working with the Council members and the staff at TTS headquarters to continue to advance the goals and missions of TTS on a global scale.


Megan Sykes, M.D., is the Michael J. Friedlander Professor of Medicine and Professor of Microbiology & Immunology and Surgical Sciences (in Surgery) at Columbia University. She is the founding Director of the Columbia Center for Translational Immunology (CCTI) at Columbia University, Director of Research for the Transplant Initiative at Columbia University Medical Center (CUMC) and Director of Bone Marrow Transplantation Research, Division of Hematology/Oncology at CUMC. Dr. Sykes completed her MD training at the University of Toronto in 1982, after which she completed a medical residency, then moved to the National Institutes of Health, Bethesda, MD in 1985 as a Fogarty Visiting Associate. She joined the Massachusetts General Hospital and Harvard Medical School as an Assistant Professor in 1990 and was tenured as a full Professor in 1999, then named to the Harold and Ellen Danser Chair in Surgery. She moved to Columbia University in 2010 to establish the CCTI, which now includes a thriving pre-clinical transplant program and a staff of 115 people, including 19 faculty members, 16 laboratory programs in transplantation, autoimmune disease, infection and cancer immunology and 6 core facilities.

Dr. Sykes introduced the idea that graft-versus-leukemia/lymphoma effects could be separated from graft-versus-host disease (GVHD) following hematopoietic cell transplantation (HCT) by allowing GVH-reactive T cells to expand while preventing migration to the epithelial GVHD target tissues. She showed that inflammation was a critical checkpoint for such migration, which was avoided when GVH-reactive T cells were administered after conditioning-induced inflammation had subsided in mixed chimeras. These studies led to clinical trials of non-myeloablative haploidentical HCT that achieved mixed chimerism across HLA barriers without GVHD. These results paved the way for the first clinical trials of mixed chimerism that achieved renal allograft tolerance across HLA barriers. Dr. Sykes dissected the role of intrathymic and peripheral tolerance mechanisms and pioneered minimal conditioning approaches for using HCT to achieve allograft and xenograft tolerance. Her work demonstrated that (and identified mechanisms by which) mixed chimerism achieves natural antibody-producing B cell tolerance and NK cell tolerance in addition to T cell tolerance. She developed a method of tracking the alloreactive T cell repertoire in human transplant recipients, and has used it along with other techniques to understand T lymphocyte dynamics in the graft and the periphery of human transplant recipients. Her work on xenogeneic thymic transplantation for tolerance induction led, for the first time, to long-term kidney xenograft survival in non-human primates.
Dr. Sykes has published more than 420 papers and chapters describing her work. She has served on TTS Council and has been President of the International Xenotransplantation Association (IXA) and Vice President of TTS. She has received many honors and awards, including the Wyeth-Ayerst Young Investigator Award from the American Society of Transplant Physicians (1998), the AST Basic Science Established Investigator Award (2007), the TTS Roche Award for Outstanding Achievement in Transplantation Science (Basic)(2010), and the TTS Award for Outstanding Achievement in Transplantation (Basic Science) (2014). She is a member of the Association of American Physicians, a Fellow of the American Association for the Advancement of Science and an Honorary Member of IXA. She was inducted into the Institute of Medicine of the National Academies (now the National Academy of Medicine) in 2009.



Kathryn Wood is Professor of Immunology Emerita in the Nuffield Department of Surgical Sciences, University of Oxford where she collaborates with the Transplantation Research Immunology Group (TRIG – www.nds.ox.ac.uk/trig) and the Oxford Transplant Centre. Professor Wood received her undergraduate degree from the University of Birmingham in Biochemistry and a D.Phil. from the University of Oxford after completing her thesis on the human complement system. She was elected to the Weir Junior Research Fellowship at University College, Oxford after completing her D.Phil and joined the Nuffield Department of Surgery in 1982 as a post-doctoral scientist where she established a research team investigating the mechanisms of induction of tolerance to alloantigens.

Kathryn’s current research focuses on immune regulation at the molecular and cellular level, mechanisms of rejection and immune modulation and interactions between the immune system and stem cell derived tissues. Together with her scientific and clinical collaborators Kathryn’s team has recently completed a Phase 1/2a trial investigating the safety and feasibility of using regulatory T cell therapy in living donor kidney transplant recipients translating the extensive laboratory work carried out by her own team and other groups internationally.

Professor Wood is a Fellow of The Academy of Medical Sciences and is currently the Khoo Oon Teik Visiting Professor at the National University Centre for Organ Transplantation (NUCOT), National University Hospital in Singapore (2015 – 2020). She has a strong interest in providing support and opportunities for the development of scientists and clinicians to enable them to achieve their full potential. To this end, she was instrumental in setting up the New Key Opinion Leaders meetings within TTS and was the founding Chair of the Women in Transplantation, a TTS initiative.
Her research achievements have been recognised internationally, including receiving a Gold Medal awarded by The Catalan Society of Transplantation (2011), The Maharshi Sushruta Award (2012), The Transplantation Society for Outstanding Achievement in Basic Sciences (2012), The Federa Prize (2014), and the Thomas E Starzl Prize in Surgery and Immunology (2017).
Kathryn was President of The Transplantation Society (2004-2006) and Editor of Transplantation (1992 - 2014).



During research career in experimental organ transplantation spanning four decades, first at Harvard and then UCLA, Dr. Jerzy W. Kupiec-Weglinski published over 470 papers and book chapters in the areas of lymphocyte recirculation, immune tolerance, host sensitization and organ ischemia-reperfusion injury. His early work identified the mechanisms by which IL-2 receptor antibodies modulate the immune system, and paved the way towards the clinical development of these agents for the prophylaxis of rejection. His later work largely focused on solving one of the most vexing problems in transplantation, i.e., how to improve the quality and number of donor organs. His contributions to the field fall into two principal categories: 1/ the discovery of key biological pathways responsible for innate immune-driven stress response in the transplant and allo-immune mediated graft rejection; and 2/ the development of novel therapies that control these pathways. Dr. Kupiec-Weglinski’s research for the past 30 years has been continuously funded by NIH. Currently, he serves as Director on Program Project Grant and Principal Investigator on three RO1s. Dr. Kupiec-Weglinski was a standing member of TTT Study Section at NIH; member, Board of Directors, American Society of Transplantation; and recipient of AST/Astellas Established Investigator Award. He holds the inaugural Paul I. Terasaki Endowed Chair in Surgery, David Geffen School of Medicine at UCLA.

Jay A. Fishman, M.D. is Professor of Medicine at Harvard Medical School, Director of the Transplant Infectious Diseases and Compromised Host Program at the Massachusetts General Hospital (MGH), and Associate Director of the MGH Transplant Center. Dr. Fishman completed medical school at Johns Hopkins University School of Medicine, internal medicine training and Infectious Disease Fellowship at MGH, and Fellowships in Molecular Biology and Genetics at MGH and Harvard Medical School. Dr. Fishman is a Fellow of the American College of Physicians, the American Society of Transplantation (AST), and the Infectious Disease Society of America. Dr. Fishman established the Transplant Infectious Disease Program of MGH to provide clinical care for recipients of solid organ and stem cell transplants. This unique program has trained many of the international leaders in the field. His laboratory investigates infections in xenotransplantation and viral pathogenesis in transplantation. Dr Fishman has over 300 publications. He has a special interest in molecular diagnostics and biotechnology and medical education. He is Past-President of the American Society of Transplantation. He is a frequent contributor at international symposia where he has shared his experience promoting worldwide transplant safety. He has received career achievement awards from AST and The Transplantation Society.

Born and raised in Guatemala, – a typical “developing country” – , still as a medical student, he rebelled against the fate of ESRD patients in his country who had no prospect of survival.
Having had the unique opportunity after his surgical residency to work with the late Prof. Rudolf Pichlmayr and with Prof. Hans G. Borst in Transplantation and Peripheral Vascular Surgery in Hannover, Germany, he was determined that, upon his return to his homeland, it would be his goal to make organ donation and transplantation possible, available and accessible for his fellow countrymen.
Since 1989 he has been involved in the creation of transplant legislation, education, the creation of the first large public dialysis facility, the initiation of adult and pediatric renal transplant programs at Guatemala’s impoverished health system´s hospitals, and the first successful transplants from living and deceased donors. He has for thirty years met the typical obstacles of an underserved country and its authorities, but also found the unique opportunity to work with the most outstanding colleagues and nurses, some visionary authorities and wonderful solidary people, who have shared his dream and made it possible, to serve hundreds of suffering patients.
TTS, DICG and RCIDT have been instrumental in this endeavour, and continue being strong allies, and a platform to learn and exchange experiences with peers and masters in the field.

Prof. HL Trivedi is Pro-Chancellor of Gujarat University of Transplantation Sciences and Founding Director of Smt. G R Doshi and Smt. K M Mehta Institute of Kidney Diseases and Research Center and Dr. H L Trivedi Institute of Transplantation Sciences (IKDRC-ITS), Civil Hospital Campus, Asarwa, Ahmedabad 380016, Gujarat, India. It is the largest Institute of its kind in the world with 438 beds for care of kidney disease patients. Prof Trivedi and his team has completed more than 5200 kidney transplantations including more than 700 deceased donor kidney transplantations and 400 robotic kidney transplantations at IKDRC-ITS Ahmedabad. Prof HL Trivedi and his team has performed largest number of kidney transplants in India and at present doing more than 350 kidney transplantations including more than 100 deceased donor kidney transplantation every year. IKDRC-ITS Ahmedabad is the only public sector hospital of India doing liver transplants and completed more than 300 deceased donor liver transplantations. He has trained more than 200 Nephrologists in India.
Prof HL Trivedi is a Pioneer Nephrologist, Immunologist, Transplanter and Stem Cell Researcher of India with many patents on stem cells therapy for transplant tolerance and insulin making stem cells. Prof Trivedi is the founder of the Indian Journal of Transplantation, Indian Journal of Nephrology, and Indian Society of Organ Transplantation which has played a pivotal role in development of transplantation as a science in the country. Recognizing his contribution towards development of nephrology and transplantation, he was awarded with HONORIS CAUSA (Doctor of Science) by Chhatrapati Shahuji University of Lucknow in 2009.
Prof HL Trivedi was awarded with Padma Shri for his distinguished service in kidney transplantation in India for poor patients – one of the highest civilian honours of India.


Dr. Karen Dwyer is a medical graduate from The University of Melbourne. She completed her training in nephrology at St.Vincent’s Hospital in Melbourne before completing her PhD under the supervision of Prof. Tony d’Apice. Dr. Dwyer completed post doc studies under the supervision of Prof. Terry Strom and Prof. Simon Robson in Boston. From 2006 until 2015, she was based at St. Vincent’s Hospital Melbourne, including as co-director of the Immunology Research Centre from 2011. During that time she supervised a number of PhD students, mostly nephrology trainees and mostly women. In 2015, Dr. Dwyer was appointed Prof. of Medicine and Deputy Head, School of Medicine at Deakin University, Victoria.
Dr. Dwyer was elected to the Transplantation Society of Australia and New Zealand (TSANZ) Council in 2013. She served for 6 years including as secretary from 2015. During this time, Dr. Dwyer introduced a plenary lecture on gender equity into the Annual Scientific Meeting, which has since been formalised as the annual Josette Eris Memorial Lecture; secured the Josette Eris Award which is awarded every 2nd year to an outstanding female early career member of the TSANZ; proposed a constitutional change to include gender representation on council. It should be noted that even without ratification of the constitutional change (due for voting in 2019), the current TSANZ council is represented 50:50 by men and women.
Dr. Dwyer has a received a number of awards including the Basic Science Mentor/Mentee Award The Transplantation Society twice; Ian McKenzie Prize for Outstanding Contribution to Transplantation; and Key Opinion Leader, The Transplantation Society.

Dr. Yuki Nakagawa is an Associate Professor at Division of Urology Department of Regenerative and Transplant Medicine, Graduate School of Medical and Dental Sciences at Niigata University. She is also a vice chairman of the Women in Japan Urological Association and a member of the committee for women. Presently, she serves of the Editorial Board for Japan Clinical Transplantation.
She graduated from Tokyo Women’s Medical University in 1991 and completed her training at Tokyo Women’s Medical University in Transplant Surgery. In 1995 she became board certified in General Surgery and Urology. She has been a staff surgeon at Division of Kidney Transplantation and Urology in the Department of Regenerative and Transplant Medicine at Niigata University Hospital since 1996.
Dr. Nakagawa has been involved in more than 1000 kidney transplant cases and clinical research especially in the field of ABO incompatible kidney transplantation and has participated in many clinical trials. She has published many articles in local and international scientific journals and actively participates in international congresses. She won the price for the 2014 Scientific Award from the Ministry of Education in Japan because of her enthusiastic campaign for promoting deceased organ donation in Japan. Furthermore, she is an active role model for female urologists as well as kidney transplant surgeons and contributes greatly to the Japanese Transplantation Society and Urological Association.

Dr. Carmen Pantis was born in Bihor County, Romania, in 1964. She studied at the University of Medicine and Pharmacy Iuliu Hatieganu in Cluj-Napoca in 1988. She was certified as an ICU doctor in 2009, one year after she was certified as a transplant coordinator. Dr. Pantis also holds a competence in emergency medicine, which she acquired in 1997. By the year 2007, she also managed to complete a doctorate in the field of pharmacology, further elevating her level of competence and knowledge in the field of emergency care and transplant medicine.
The main center of her activity revolves around the Clinical Emergency County Hospital in Oradea, Romania, and the Faculty of Medicine and Pharmacy within the University of Oradea. Dr. Pantis has been the Regional Transplant Coordinator in Romania since August 2016. She has participated as a speaker in a variety of workshops relating to the topic of transplantation such as the one in Ohrid, Macedonia in 2012 and GLS San Diego in the same year. Additionally, she has held the position of President of the Bihor Medical College since December 2011 and is also a lecturer in the field of Surgery and ICU at the Faculty of Medicine and Pharmacy in Oradea.
Additionally, Dr. Pantis has well over 100 significant written and oral contributions to medical papers and medical conferences in a large variety of domains including pharmacology, surgery, transplant medicine and ICU care.


Rebeca Arroyo-Hornero received a Young Investigation Award for her research in regulatory T cell therapy for transplantation. Rebeca completed her work under the supervision of Dr. Joanna Hester and Dr. Fadi Issa, principal investigators at the Transplantation Research Immunology Group at the University of Oxford. Regulatory T cells are currently being tested as a cellular therapy in transplant patients aiming at promoting transplant tolerance and reducing levels of immunosuppression chemotherapy. In this particular study it was found that expression of CD70 identifies unstable regulatory T cells that lose their suppressive abilities and, instead, induce a pro-inflammatory microenvironment and T cell proliferation. All suggests that modulation of the CD27/CD70 pathway may allow for the generation of regulatory T cells with enhanced suppressive properties after in vitro expansion.
Rebeca is currently completing her PhD at the University of Oxford, studying cellular therapies for transplantation. She is interested in discerning how regulatory T cells suppress the immune response and finding new methods for controlling the stability and suppressive activity of regulatory T cells to produce an effective clinical cellular therapy.

Dr. Yarl Balachandran was chosen as a recipient of the Young Investigator Award for her work on the viral and host cell genomic alterations found in Post-Transplant Lymphoproliferative Disorder (PTLD). She completed her work as a Postdoctoral Scholar in Transplantation Surgery under the supervision of Drs. Carlos Esquivel, Sheri Krams, and Olivia Martinez. Her findings showed that key gain-of-function mutations associated with PTLD and detected in blood and cell lines are also found in the primary tumor, suggesting a role in tumorigenesis and great potential as biomarkers.
Dr. Balachandran received her undergraduate degree from Columbia University. She completed her thesis in Nobel Laureate Dr. Eric Kandel’s laboratory, studying neurotrophins and their role in learning and memory. She earned her medical degree from Harvard Medical School. For her thesis, she characterized novel therapeutics in patient-specific stem cell models at Massachusetts General Hospital. Currently, she is a Surgical Resident at Stanford University Medical Center. She was recently invited to present her findings at the National Institutes of Health and American Transplant Congress.

Florencia Bonisconti received a Young Investigator Award for her work in “Clinical Utility of a modified qRT-PCR for Trypanosoma cruzi detection in transplant patients”. Florencia completed his work in the laboratory of molecular diagnosis in Hospital Privado Universitario de Cordoba in Argentina. The laboratory started its work in Chagas Disease (CD) with the first heart transplants in recipients with CD. Since then, there was need to develop a molecular method that allows these patients’ follow-up in post-transplant. First the laboratory develops a conventional PCR, after that perform a quantitative methodology from the PCR published by Piron et al. in 2007. In this work, the authors showed the immunosuppressed patients’ follow-up with CD and the importance of an early diagnosis of Trypanosoma cruzi reactivation or de novo infection in these patients using this method.
Florencia Bonisconti is a Biochemistry MS. She has completed others projects receiving the TID award in 2015. Nowadays, she is writing the paper of this work for its publication and plans to start a PhD in Biochemistry.

Xiaoyong Chen received a Young Investigator Award for his work on identification of the CD23+CD43+ regulatory B cells (Breg cells). Chen completed his work under the supervision of Prof. Andy Peng Xiang, the Director of Center for Stem Cell Biology and Tissue Engineering at Sun Yat-Sen University. Results from his work identify a novel regulatory B cell population characterized as CD23 and CD43 phenotypic markers could be induced by mesenchymal stem cells (MSCs). The CD23+CD43+ Breg cells significantly inhibited the inflammatory cytokine secretion and proliferation of T cells through an IL-10-dependent pathway. These MSC-treated Breg cells may be an important regulatory B cell subset responsible for the ability of MSCs to control inflammation-related diseases or conditions, including transplantation.
Xiaoyong Chen obtained a PhD degree on June 2015, and now he is a lecturer at Sun Yat-Sen University. He is interested in understanding the therapeutic mechanisms of MSCs. One of his previous study reported that MSC infusions improve refractory chronic graft versus host disease (GVHD), a severe complication after allogeneic hematopoietic stem cell transplantation, through an increase of CD5+ regulatory B cells producing interleukin 10(IL-10). For this work, he received a Young Investigator Travel Awards in the 12th Congress of the Cell Transplant Society. He is the first author of 6 peer-reviewed publications on Leukemia, Mol Ther, Cell Mol Immunol and etc. In the future he will continue his work on immunomodulation of MSCs in inflammation-related diseases or conditions, and he will focus on their cellular and molecular mechanisms.

Fang Kuan Chiou received a Young Investigator Award for his work on “Poorer long-term survival associated with monomorphic post-transplant lymphoproliferative disorder after solid organ transplantation in children”, which was completed under the mentorship of Dr Girish Gupte and Dr Sue Beath at the Liver Unit (including small bowel transplantation) at Birmingham Children’s Hospital in the United Kingdom. The study shows that monomorphic PTLD is associated with more advanced disease and significantly poorer prognosis compared to other histologic subtypes of PTLD, but improved remission and survival rates are achieved with the introduction of EBV-specific cytotoxic T-lymphocyte therapy to the treatment protocol.
Fang Kuan Chiou is a paediatric gastroenterologist with a special interest in paediatric hepatology and transplantation at KK Women’s and Children’s Hospital in Singapore. He trained as a Clinical Fellow in Hepatology at the Liver Unit at Birmingham Children’s Hospital from 2016 to 2017. He aims to enhance the field of paediatric hepatology and further develop liver and intestinal transplantation services in Singapore.

Helong Dai received a Young Investigator Award for his work on a novel technique for en bloc kidney transplantation from infant donors with extremely low body weight. Dr. Dai and his colleagues completed this work in The Second Xiangya Hospital of Central South University, China. Eight cases of en bloc kidneys from deceased infant donors younger than 5 months with low body weight (1.9-4.9 kg) were transplanted into 4 pediatrics and 4 adults. By using the donor’s distal abdominal aorta as an outflow tract, this novel en bloc kidney transplantation significantly decreased the incidence of vascular thrombosis post-transplantation and effectively expanded the organ donor pool.
Dr. Dai received his MD and PhD in China, and then began his surgical residency at The Second Xiangya Hospital. Currently, he is a post-doctoral fellow at the Thomas E. Starzl Transplantation Institute, University of Pittsburgh. He is a member of TTS, AST and IPTA. His research focuses on the mechanism of long-term graft survival in kidney transplant patients; the role of mTORC2 in dendritic cells and allograft rejection; regulatory dendritic cells and their therapeutic potential.

Nicole De La Mata received a Young Investigator Award for her work on stroke mortality in kidney transplant recipients. In collaboration with Professor Angela Webster and Associate Professor Patrick Kelly, a retrospective population-based cohort of all kidney transplant recipients in Australia and New Zealand was established using data linkage. This study found that stroke mortality was substantially higher among kidney transplant recipients compared to the general population, particularly for young people and women. Also, a greater risk of stroke death was associated with earlier year of transplant, pre-existing cerebrovascular disease and graft failure.
Nicole is an early-career biostatistician working at the University of Sydney School of Public Health in Australia. She has previous experience in managing and utilizing large observational cohorts to evaluate patient outcomes and influence health policy. Her current research focuses on mortality and health outcomes in people with end-stage kidney disease, living kidney donors and organ transplant recipients. She has a particular interest in statistical methods of survival data, such as modelling in the presence of competing risks and modelling relative survival.

Reinier de Vries, MD, received a Young Investigator Award for his work on supercooling of human livers to extend the preservation time for transplantation. Together with his research team under supervision of Dr. Korkut Uygun he for the first time demonstrated the feasibility of subzero human organ preservation, significantly extending the ex vivo life of the organ with a combination of supercooled ice-free storage and recovery with subnormothermic machine perfusion.
Reinier de Vries, received both his BSc in mechanical engineering and MSc in medicine cum laude from the Technical University Delft in 2013 and The University of Amsterdam in 2018 respectively. As a postdoctoral research fellow at the Center for Engineering in Medicine of Massachusetts General Hospital and Harvard Medical School he dedicates his work to bringing technological innovations to the patients' bedside. In particular, his research involves extended organ preservation and strategies to improve organ viability to expand the donor pool for transplantation.

Dr. Su Kah Goh received a Young Investigator Award for his work on "Donor-specific cell-free DNA as an emerging biomarker of organ rejection after liver transplantation”. Su Kah completed his research under the supervision of Prof Chris Christophi, Head of the Hepato-pancreato-biliary Unit (University of Melbourne) at the Austin Health, Australia and A/Prof Alexander Dobrovic, Group Leader of the Translational Genomics and Epigenomics Laboratory at the Olivia Newton-John Cancer Research Institute, Australia.
His study highlighted the use of a cost-effective and simple approach to quantify donor-specific cell-free DNA in transplantation. The exploratory use of this non-invasive approach was shown to be effective for identifying recipients with biopsy-proven acute rejection after liver transplantation.
Su Kah is currently completing the submission of his PhD thesis. He has also recently recommenced surgical training to be a general surgeon. In recognition for his endeavours, Su Kah has received numerous awards, scholarships from the Royal Australasian College of Surgery, and small project grants from the Australia New Zealand Hepatic, Pancreatic and Biliary Association to expand his research interests in the field of transplantation at the Austin Health.

Christian Heim received a Young Investigator Award for his work on small molecule tyrosine kinases as preventive strategy against cardiac allograft vasculopathy. In the last years, Dr. Heim has performed several studies on treatment options of cardiac allograft vasculopathy in experimental mouse transplant models. In this particular study it was found that nintedanib reduced different growth factor receptors and hereby ameliorated the development of allograft vasculopathy in a mouse aortic transplant model.
Dr. Christian Heim is consultant cardiac surgeon at the University Clinic Erlangen and team leader of the experimental cardiac laboratory. He studied medicine at the University of Erlangen/Germany, Wellington/New Zealand, and Edmonton/Canada. He has completed a doctoral thesis on transplant immunobiology under the supervision of Prof. Ensminger, now Director of Cardiothoracic Surgery in Lübeck/Germany. After finishing his specialty surgical training for cardiothoracic surgery, Dr. Heim subsequently wrote his “Habilitation” thesis on thoracic organ transplantation at the Department of Cardiac Surgery Erlangen/Germany (Head: Prof. Weyand).

Charlotte Lee received a Young Investigator Award for her work on The Anti-Inflammatory effect of Alpha-1 antitrypsin in Hepatocyte Transplantation. Charlotte completed her work under the supervision of Dr Emer Fitzpatrick and Professor Anil Dhawan at the Institute of Liver Studies at King’s College Hospital, London. This work involved a collaboration with Professor Maria Koulmanda at the Beth Israel Deaconess Medical Center at Harvard Medical School. This study showed in an ex vivo blood perfusion system that alpha-1 antitrypsin inhibited coagulation activation and decreased pro-inflammatory cytokine expression when hepatocytes were added to ABO-matched blood. In a rat model of hepatocyte transplantation, treatment with alpha-1 antitrypsin significantly improved engraftment of cells at 24 and 48 hours. This work is now undergoing pre-clinical work in a rat model of metabolic liver disease before the start of a clinical trial which is due to commence shortly.
Charlotte Lee completed her PhD in September 2017 from King’s College London and is now in her first post-doctoral position in the same group. She has been involved in a number of projects throughout her PhD, one of which led to an Early Career Researcher Grant Award to allow her to investigate the potential of using cell-free DNA to track engraftment of hepatocytes. She has also done considerable work investigating the potential of neonatal donors for hepatocyte transplantation which led to a publication in Liver Transplantation.

Xiaoqian Ma received a Young Investigator Award for her work on finding Cord blood derived regulatory macrophages (Mreg) – an alternative source for Mreg-based cell therapy in transplantation. Xiaoqian completed her work under the supervision of Prof. Wei Wang, the Director of Institute for Cell Transplantation and Gene Therapy, the Third Xiangya Hospital of Central South University (CSU, China). Results of the study confirm that CB-derived Mreg has similar yield and phenotype with adult peripheral blood derived Mreg. Compared to their APB-Mreg, CB-Mreg were more potent in suppression of the allogeneic response in vitro due, at least in part, to their upregulated IDO expression. All together demonstrates CB-derived Mreg as a potential source for large-scale preparation of human Mreg to meet the demands for clinical cell therapy in immunomodulation in transplantation.
Xiaoqian Ma was awarded her associate professorship in 2016 from the Central South University. She has completed multiple projects, receiving several grants and various awards in recognition for her work. In 2012, she got the first funding from the National Natural Science Foundation of China, which has helped her to start her research on transplantation. She was also supported by Natural Science Foundation of Hunan Province, China in 2017 and the Project of Health and family planning commission of Hunan Province in 2016. She received a TTS-CTS 2015 Scientific Award in Melbourne, Australia for the contribution to transplantation immunology.

Dr. Berenice Mbiribindi received a Young Investigator Award for her work on NK cell recognition of peptides encoded by EBV latent cycle proteins. Dr. Mbiribindi is currently a Postdoctoral Fellow under the mentorship of Prof Sheri M. Krams, the Director of Transplant Immunology at Stanford University School of Medicine. Since joining the Transplant Immunology Lab in the Department of Surgery at Stanford, Dr. Mbiribindi has focused on understanding how to harness NK cells to treat EBV infections. Epstein–Barr virus (EBV) infects more than 90% of adults worldwide and is associated with several malignancies, including post-transplant lymphoproliferative disorder (PTLD). She has demonstrated that latent cycle proteins from EBV can encode for peptides that bind to HLA-E. These EBV peptide: HLA-E complexes may be important in the elimination of cells infected with EBV. As NK cells are generally resistant to immunosuppression, these findings can lead to improvements in therapeutic strategies to control EBV diseases, including PTLD, post-transplant.
Dr. Mbiribindi received her PhD in Immunology and Infection from Southampton University (UK) working on NK cells. She is currently involved in several projects focusing on NK cells and she has received awards in recognition for her work. Most recently, she was awarded two fellowships from the Stanford Child Health Research Institute (CHRI) and The Transplant and Tissue Engineering Center of Excellence (TTE).

Hiroyuki Ogasawara received a Young Investigator Award for his work on the Comparison of the Transplant Efficiency between Intraportal and Intrasplenic Procedures in Hepatocyte Transplantation. Hiroyuki completed his work under the supervision of Dr. Masafumi Goto, the Professor of Division of Transplantation and Regenerative Medicine and Dr. Takashi Kamei and Dr. Michiaki Unno, the Professor of Department of Surgery at the Tohoku University. In hepatocyte transplantation, intraportal injection is regarded as the current standard procedure. However, some previous studies showed intrasplenic approach is more efficient in hepatocyte engraftment. Therefore, we examined the transplant efficiency between intraportal and intrasplenic procedures in hepatocyte transplantation using analbuminemic rat, immunohistochemical analyses (BrdU), and in vivo imaging system. The study showed that the intraportal procedure is more efficient than the intrasplenic procedure. Furthermore, the graft function in the intrasplenic group was proved to be almost entirely achieved by hepatocytes that have migrated to the liver, suggesting that hepatocyte engraftment is more dependent on the transplant-site environment than the transplant procedure.
Dr. Ogasawara works at the Department of Surgery, Tohoku University Hospital and his field of specialty is hepatic surgery and liver, kidney and pancreas transplantation. He is currently completing his Ph.D. from the Tohoku University.

Brenda Rosales received a Young Investigator Award for her work on cancer mortality in kidney transplant recipients using a national Australian and New Zealand transplant registry (ANZDATA) and respective national mortality registers. Brenda completed her work under the supervision of Prof Angela C Webster, Professor of Epidemiology at the Sydney School of Public Health in University of Sydney, and Senior Specialist in Nephrology at Westmead Hospital, NSW Australia. In this bi-national cohort, it was found that kidney transplant recipients have over three times the excess mortality of an age, sex and calendar year matched general population, and that this has remained unchanged between 1980 and 2013. There was a great degree of variability of excess mortality by cancer site, with the highest excess mortality in non-melanoma skin cancers (over 50 times that of the general population). These results demonstrate the risk of death for kidney transplant recipients, compared to the general population and may inform future research in site-specific screening strategies.
Brenda Rosales is currently completing her PhD at the Sydney School of Public Health, University of Sydney. Her work is informed by her four years’ experience as a Transplantation Scientist at the NSW Transplantation and Immunology Services, Australian Red Cross Blood Service and as a Research Assistant investigating blood biomarkers for breast cancer at the Surgery and Cancer Department, Imperial College London. This is her first international award.

Akhil Sharma received a Young Investigator Award for his work on Pro-Inflammatory B Cells predicting progressive early minimal renal allograft inflammation and its association with poor long term renal allograft outcomes. Akhil completed his work under the supervision of Dr. David M. Rothstein, Professor of Surgery, Medicine, and Immunology as well Pittsburgh Steelers Chair in Transplantation at the University of Pittsburgh, School of Medicine. Results of their study demonstrate that early renal allograft inflammation was associated the development of late acute rejection. Furthermore, patients who have early renal allograft inflammation that progresses to late acute rejection were associated with worse long term clinical outcomes. Lastly, Pro-Inflammatory B cells may help identify patients with early renal allograft inflammation at risk for poor long term clinical outcomes.
Akhil Sharma completed his M.D. from Wayne State University School of Medicine. He subsequently completed his Internal Medicine Residency, General Nephrology Fellowship, and Transplant Nephrology fellowships at University of Pittsburgh. He currently is a Clinical Instructor of Medicine in the Renal-Electrolyte Division and continues to work in Dr. David M. Rothstein’s lab at the University of Pittsburgh.

Dr. Ashley Suah received a Young Investigator Award for her project entitled ‘Acquired resistance to transplantation tolerance as a result of prior pregnancy requires B cells.’ Ashley completed her work under the mentorship of Dr. Anita Chong, Director of the Transplant Immunology Research Center and Professor of Surgery at the University of Chicago. Results of Ashley’s studies provide new insights into the mechanism of pregnancy-induced sensitization by demonstrating a necessity for B cells in preventing subsequent induction of fetal/allograft-specific transplantation tolerance.
Following the completion of her second clinical year of General Surgery training at the University of Chicago, Ashley completed a two year research fellowship in Dr. Chong’s lab. She recently returned to the clinic, and is currently in her third year of residency. During her two year research fellowship, Ashley received various awards for her work related to the immunological effects of pregnancy, including the American Society of Transplant Surgeons Resident Scientist Scholarship, an American Transplant Congress Young Investigator Award, and the Advances in Organ Transplantation FellowsChoice Award. While in the lab, she also completed a Medical Ethics Fellowship at the MacLean Center at the University of Chicago.

Patrick Trotter received a Young Investigator Award for his work on the use of kidneys from donors who die following ligature asphyxiation and there effect on transplant outcomes. Patrick completed his work under the supervision of Professor Christopher Watson and Professor J Andrew Bradley at the Department of Surgery, University of Cambridge. The results of the study demonstrated that patient outcomes following transplantation of kidneys from donors who died following ligature asphyxiation were comparable to those who received kidneys from all other donors.
Patrick Trotter has just completed his PhD at the University of Cambridge investigating the multifactorial role that infections in organ donors play in organ transplantation and was the recipient of the TTS-TID travel award in 2017. Patrick has completed multiple projects during his PhD, and has received various awards for his work and has recently started working at Royal Papworth Hospital, Cambridge, United Kingdom.

Marieke van der Zwan received a Young Investigator Award for her work on the efficacy of rabbit anti-thymocyte globulin for glucocorticoid resistant acute kidney allograft rejection. Marieke works under the supervision of Dr. D.A. Hesselink, Dr. M.C. Clahsen-van Groningen and Prof. Dr. C.C. Baan of the Rotterdam Transplant Group (Erasmus Medical Center, The Netherlands). Her current project focusses on the long-term outcomes and adverse events of T cell depleting therapy (rabbit antithymocyte globulin and alemtuzumab) for glucocorticoid resistant acute kidney allograft rejection. Besides, she investigates biomarkers for acute kidney allograft rejection in belatacept-treated patients.
Marieke van der Zwan completed her M.D. and MSc Molecular Medicine in Erasmus Medical Center (Rotterdam, The Netherlands). Currently, she is a resident nephrology and a PhD student at the Nephrology & Transplantation Laboratory at Erasmus Medical Center. In addition, she is editor for The Netherlands Journal of Medicine and an active member for the Dutch Federation of Nephrology.

Karen Waller received a Young Investigator Award for her work on the residual risk of blood borne viruses among increased risk donor referrals in Australia. This work was completed under the supervision of Professor Angela Webster, Epidemiologist at the University of Sydney and Transplant Nephrologist at Westmead Hospital, and Associate Professor Kate Wyburn, Transplant Nephrologist and Head of Kidney Transplantation at Royal Prince Alfred Hospital. Karen’s work showed that in the setting of negative testing, the residual risk of HIV among increased risk groups remains low in absolute terms. Interestingly, the risks calculated in Australia are lower than those seen in international studies (USA, Canada).
Dr Waller is undertaking a MPhil through the University of Sydney, with research focussing on the impact of blood borne viruses on transplantation in Australia. Her projects have received recognition in the form of Young Investigator Awards at national conferences, including the Annual Scientific Meeting of the Transplantation Society of Australia and New Zealand this year. She hopes to convert her research into a PhD in 2019. Karen is also a Basic Physician Trainee at Royal Prince Alfred Hospital, Sydney, Australia, having successfully passed her written and clinical examinations in Adult Medicine through the Royal Australian College of Physicians this year.

Casey Ward received a Young Investigator Award for his work on the Preservation of Pancreatic Islet Grafts in the Extra-Hepatic Space with Novel Parathyroid Gland Co-transplantation. Casey completed his work under the supervision of Dr. Peter Stock, co-director of Pancreatic Islet Cell Transplant Program and Dr. Qizhi Tang, director of the Transplantation Research Laboratory at the University of California- San Francisco (UCSF). Results of the study confirm that islet transplantation can cure type 1 diabetes; however, multiple donors are needed due to extensive perioperative loss of islets away from their native blood supply. In comparison, parathyroid gland (PTG) autotransplantation in the subcutaneous (SQ) and intra-muscular (IM) sites is an established surgical procedure. In this study, we exploited the neoangiogenic and paracrine hormonal factors made by PTG for complete preservation of mature islet and stem cell grafts leading to reversal of diabetes with previously unattainable minimal mass of islets.
Casey Ward completed his M.D. from Oregon Health and Science University School of Medicine and is currently completing his General Surgery residency training at UCSF with a plan to pursue Transplant Surgery fellowship in the future. He has received multiple awards for his innovative research in islet and parathyroid gland transplantation. Furthermore, the pre-clinical data obtained from this study has led to the initiation of a Phase I/IIa clinical trial at UCSF.

Dr. Cheng Yang received a Young Investigator Award for his work on Prediction of Renal Allograft Chronic Rejection using a Model Based on Contrast Enhanced Ultrasonography. Dr. Yang completed his work under the supervision of Prof. Tongyu Zhu and Prof. Wanyuan He. In this study, Dr. Yang evaluated the application of contrast-enhanced ultrasonography in the assessment of renal allograft rejection by establishing and validating a new noninvasive index to predict chronic rejection (CR). The AUROC of this simple index is as high as 0.89. Two cut-off values were chosen to identify the absence (less than 0.36) and presence (greater than 0.70) of renal allograft CR. Using these two cut-offs, about 70% patients could be correctly diagnosed, with over 90% accuracy. The new index provides a new diagnosis model for CR.
Dr. Yang received her M.D. and PhD. from the Fudan University in Shanghai. He then completed his surgical residency at Zhongshan Hospital, Fudan University. During the PhD training, he visited University Leicester and collaborated with Prof. Bin Yang and Prof. Michael Nicholson on the research of acute and chronic kidney injury. He is now working as a renal transplant surgeon in Department of Urology at Zhongshan Hospital. Besides TTS, Dr. Yang is also a member of AST. During 2012-2016, he received 6 awards from TTS and AST, such as Young Investigator Award and Yong Innovator Award etc. His research interests are: acute kidney injury, rejection and tolerance, immune regulation in transplantation and cell apoptosis/necroptosis.

Yuanfei Zhao received a Young Investigator Award for her work on memory Tregs with the antigen specificity in the long-term islet xenotransplantation animal models. Yuanfei is working under the supervision of Professor Philip O'Connell, the Director of the Centre for Transplant and Renal Research at the Westmead Institute for Medical Research. He was the former president of TTS from 2014 to 2016. In this study, it was found that the highly-selected population of Tregs from long-term tolerant xenotransplantation model had the more immunosuppressive function after adoptive-transfer beyond 100 days. Results currently suggest that the memory Tregs may have the potential to be one of subsets of Tregs used for cell-based therapy in xenotransplantation.
Yuanfei Zhao completed her medical degree in China, and she is currently completing her PhD in the field of transplant immunology at the Sydney University School of Medicine. She has also undertaken a project on human regulatory macrophages in allotransplant assay. Her major research interests are the cellular therapies in the kidney and islet transplantations. In the future, she will continue her work on immunological therapy after kidney and islet transplantation, and she will focus on the identification of antigen-specific Tregs to improve the efficiency in applications.


Mr. Ibrahim Adam received a Mentee-Mentor award for his work in antibody response to A-antigen in the setting of syngeneic, allogeneic, and xenogeneic stimulation. Mentored by Dr. Lori J. West, her work is investigating the cells and molecules participating in immunity and tolerance in the setting of ABO-incompatible heart transplantation (ABOi-HTx). This work is aimed to expand donor pool and immune tolerance that currently is limited to infants and young children.Understanding how ABO-mismatched heart transplants are accepted or tolerated will allow strategies to be developed to extend the ABOi-HTx beyond infancy, thus having an impact on the health of many patients with end-stage heart disease. Our overall goal is to define the processes by which the immune system changes after transplant allowing acceptance of ABO-mismatched hearts, rather than rejection.
Mr. Adam received a BSc in Medical Lab. Sciences and an MSc in Genetics and Molecular Biology, from the University of Khartoum in Sudan. He has worked as a Medical Lab Technologist in Sudan and in the National Health Laboratory, Khartoum, Sudan. He has also worked with Doctors without Borders/Medecins Sans Frontieres (www.MSF.org) in South Sudan. Mr. Adam is currently a PhD candidate in Immunology at the Dept. of Medical Microbiology and Immunology at the University of Alberta. His future interest is to work in a diagnostic laboratory in the field of transplantation immunology.

Avery Lam received a Mentee-Mentor Award with Dr. Megan Levings for his work on tissue-reparative human regulatory T cells (Tregs). He found that human Tregs in blood and multiple tissue types produced the tissue-repair factor amphiregulin, but that this was neither a unique feature of Tregs nor selectively upregulated in tissues. Furthermore, the IL-33/ST2 pathway did not control amphiregulin expression in human Tregs, in contrast to findings in mice. Engineered ST2+ Tregs may promote tissue repair innately, and future work aims to better understand and harness this function in the context of cell therapy.
Avery is a Ph.D. candidate in Experimental Medicine at the University of British Columbia. He received his B.Arts Sc. in the Arts & Science Program with Biochemistry at McMaster University in 2015. In Dr. Levings’ lab, his thesis work focuses on defining the signaling pathways governing human Treg stability and function. During his undergraduate degree, he worked in cancer therapeutics and immunotherapy with Drs. David Spaner (University of Toronto) and Brian Lichty (McMaster University).


Dr. Manish Ramesh Balwani received a Mentee-Mentor award for his work on “Knowledge Regarding Brain Death and Organ Donation Laws Among Medical Students”. Mentored by Prof. Pankaj Shah, this work evaluates the knowledge among medical students regarding organ donation & its laws. It was found that there was enough scope to improve the understanding among medical students regarding early identification of brain death. Better knowledge and awareness about organ donation laws will help in early identification of cadaver which will increase cadaver donation indirectly. A thorough topic should be covered in MBBS syllabus with in hand practical involvement of students while carrying out organ donation and allocation activities. This will help in removing fears and hesitancy among medical fraternity regarding organ donation.
Dr. Manish Balwani received his MD in Internal Medicine from SMS Medical College, Jaipur, India and later completed his DM Nephrology speciality training under the guidance of Prof. H L Trivedi & Prof. Pankaj Shah from IKDRC-ITS, B. J. Medical College, Gujarat University, Ahmedabad, India. He is currently a Consultant Nephrologist & Transplant Physician at Jawaharlal Nehru Medical college, Sawangi, India & also has Nephrology Clinic at Saraswati Kidney Care Center, Nagpur, India. His research interests include the preventive nephrology, organ transplantation policies, post renal transplant drug therapeutics monitoring & post transplant hemolytic uremic syndrome evaluation.

Dr. Sonia Mehrotra received Mentee-Mentor Award for the study on Pharmaco-dynamics of CSA and Tacrolimus and its Effect on Antiviral Drugs in HCV Positive Kidney Transplant Recipients with Dr Raj Kumar Sharma as mentor.The aims of this pilot study were to assess the effect of interferon free sofosbuvir and ribavirin combination regimen used to treat chronic hepatitis C viral (HCV) infection in kidney transplant recipients on pharmacokinetics of calcineurin inhibitor (CNI ) drugs. Direct acting antiviral drugs can affect drug levels of CNIs by increasing their clearance. Pharmacokinetics of CNI drugs need to be studied while transplant patients are on Sofosbuvir to see effect on AUC as decreased exposure to immunosuppression can precipitate rejection. With sofosbuvir therapy and viral clearance, there was reduction in CNI levels due to increased clearance of the CNI drugs, which is shown by the AUC measurements. This could be important for patients at high risk for rejection.
Dr. Mehrotra is Lab-in-Charge of Renal Transplant Lab at department of Nephrology Sanjay Gandhi Post Graduate Institute of Medical Sciences India. Her PhD topic was on “Vitamin D metabolism in chronic kidney disease patients undergoing renal transplantation and its effect on post-transplant outcomes”. She was awarded the International Society of Nephrology (ISN), ANIO Advanced Apprenticeship in 2015 on histocompatibility and immunogenetics. She received The Transplantation Society (TTS) Transplantation Science Mentee Mentor Award in 2016. Dr. Mehrotra has authored or co-authored over 15 research publications, and co-authored in four different book chapters.
Dr. Mehrotra is an active member of The Transplantation Society (TTS), American Society of Histocompatibility and Immunogenetics (ASHI), and the Indian Society of Organ Transplantation (ISOT).

Dr. Mohan Puna Patel received a Mentee-Mentor award for his work on “Prediction of Tacrolimus Drug Dosing and Metabolism based on CYP3A5 polymorphism in Indian renal transplant recipients”. Mentored by Prof. Manoj Gumber, this work assesses the impact of CYP3A5 polymorphism on dose requirements & metabolism of tacrolimus in renal transplant recipients. The study found that polymorphism does influence pharmacokinetics of tacrolimus significantly. Author recommends pre-transplant CYP3A5 genotype analysis to be done for better individualization of tacrolimus dosing and also helps in avoiding drug toxicity related allograft dysfunction in post-transplant period. Future long term studies are planned to demonstrate epidemiological status of CYP3A5 polymorphism and also help to optimize tacrolimus dosing.
Dr. Mohan Patel got his MD in Internal Medicine from Govt. Medical College, Nagpur and subsequently completed his super-specialty training, DM Nephrology under the guidance of Prof. Dr. H L Trivedi & Prof. Manoj Gumber from IKDRC-ITS, B. J. Medical College, Gujarat University, Ahmedabad, India. He is currently working as Consultant Nephrologist & Transplant Physician with Apollo Hospitals, Nashik, India. His research interests include post transplant drug level monitoring, developing cadaveric kidney transplantation and studying post transplant infections.

Asuka Tanaka received a Mentor-Mentee award for her work entitled “Donor conditioning with recipient-derived PD-L1/PD-L2-expressing B cells prevents lethal acute GVHD in a fully allogeneic mouse model”. She investigated the immune-regulatory roles of B cells as regulatory antigen-presenting cells targeting allo-reactive T cells. Her laboratory reported that MHC classⅡ+CD80+CD86+PD-L1+PD-L2+ B cell subclass are exclusively part of the CD5+ B-1a cells that are located in the peritoneal cavity. These PD-L1/PD-L2 B-1a cells expressed surface molecules necessary for efficient Ag-presentation to T cells together with the apoptosis-inducing ligand, potentially imparting their tolerogenic potential upon alloantigen recognition of T cells. In this study, she focused the mechanisms of immune-tolerance induced by these unique B cells in murine allogenic bone marrow transplantation.
Dr. Tanaka received her M.D. from the Tottori University, and she has gained clinical experience as a transplant surgeon. She is now a Ph.D. student in Gastroenterological and Transplant Surgery, Hiroshima University, Japan. Under Dr. Hideki Ohdan and Dr. Yuka Tanaka’s mentorship, Dr. Tanaka’s research is aimed at inducing immune tolerance by those immune-regulatory B cells targeting allloreactive T cells in allo -transplantation.

Jesus M. Sierra Parraga received a Mentor-Mentee award for his work studying the mechanisms behind the immunoregulatory properties of mesenchymal stem cells. Mentored by Martin J. Hoogduijn, his work examined the immunological mechanisms determining the fate of infused MSC and the immunomodulatory response associated with it. Recognition of MSC by the innate immune system induced phenotypical and functional changes in monocytes, which subsequently modulated cells of the adaptive immune system. It was found that monocytes play a crucial role in mediating, distributing and transferring the immunomodulatory effect of MSC.
Mr. Sierra Parraga completed his bachelor in Biotechnology from the Polytechnic University of Valencia (Spain) and got his Master´s degree in Translational Biomedical Research from Cordoba University (Spain). He is currently a PhD student at the Internal Medicine department of the Erasmus Medical Center in Rotterdam (The Netherlands). Under the supervision of Dr. Martin Hoogduijn, his research is focused on the therapeutic effect of MSC and how these cells interact with the host cells to understand the underlying mechanisms of MSC therapeutic effect and to improve outcome of MSC therapy.

María García-Conde received a Mentee-Mentor Award for her work related to liver transplantation from donation after circulatory death type IIA (DCD IIA), mentored by Dr. Iago Justo. This work focuses on the DCD IIA liver transplantation programme initiated in University Hospital 12 de Octubre over 10 years ago, evaluating the changes implemented in both candidate selection and donor maintenance optimisation over time, and how these modifications impact liver transplant results. Risk factors related to decreased patient survival are also evaluated, with the intention to approach an ideal DCD IIA graft-recipient allocationand minimise complications.
Ms. García-Conde got her MD in medicine from Complutense University in Madrid (Spain), completed her Surgery residence at University Hospital 12 de Octubre and is currently preparing to obtain her PhD on medical-surgical science fromComplutense University. Her research is focused on high risk liver grafts and the underlying mechanisms behind the complications associated with donation after circulatory death.


Selin Akad received a Mentee-Mentor Award for her work entitled “Impact of MMP2, MMP9 and TIMP2 Gene Polymorphisms on Allograft Rejection in Pediatric Renal Transplant Recipients''. Prof.Dr. Feride Sahin from Baskent University Faculty of Medicine Department of Medical Genetics mentored Selin Akad during her work. Selin Akad’s project was about the understanding of the effects of Matrix Metalloproteinase (MMP) genes on allograft rejection in pediatric renal transplant recipients. For this purpose, she analyzed the functional polymorphisms of MMP2, MMP9, and TIMP2 genes which effect their expression, and tried to establish an association between any one of the polymorphism and allograft rejection. This was a retrospective, single-center, cross-sectional study on pediatric renal transplant patients. A total of 68 kidney transplant recipients and 58 controls were enrolled in the study. As a result, they found a statistically significant difference between the allele frequencies of the functional polymorphisms of MMP2, MMP9, and TIMP2 genes in the pediatric renal transplant and control patients as well as in allograft rejection and non-rejection patients. According to their results, they suggest that MMPs and their tissue inhibitors may be important predictive biomarkers for monitoring renal transplant recipients.
Ms. Selin Akad completed her Master of Science Degree at Baskent University Faculty of Medicine, Department of Medical Genetics. She is currently a Ph.D. student at Baskent University Faculty of Medicine, Department of Medical Genetics. She interested in genetic mechanisms of diseases as study area. As molecular mechanisms underlying allograft rejection are important during clinical management and follow up of patients, Selin is interested in transplantation studies in order to elucidate these mechanisms.

Sam Adhikary received a Mentee-Mentor Award for his work investigating the effect of post-transplant cyclophosphamide (PTCy) treatment on the development of graft-versus-host disease (GVHD) in a humanised mouse model. Under supervision of Dr. Debbie Watson and Associate Professor Ronald Sluyter at the Illawarra Health and Medical Research Institute (IHMRI), University of Wollongong (UOW), Australia, immunodeficient mice were injected (i.p.) with human immune cells to induce GVHD and subsequently injected with 33mg/kg PTCy or saline. Mr. Adhikary found that PTCy significantly reduced the reduction of GVHD in humanised mice, with PTCy-injected mice demonstrating reduced weight loss and GVHD clinical score, and increased overall survival compared to saline-injected mice. PTCy-injected mice also demonstrated an increased hCD4+:hCD8+ T cell ratio and a reduction in the relative hIL-17 expression in the small intestine, an important cytokine in GVHD development. Whilst PTCy did not completely prevent GVHD, these findings will allow further study in combinational therapy alongside PTCy to prevent GVHD long-term in this model.
Mr. Adhikary graduated with a Bachelor of Medical Biotechnology (Advanced) (Hons. Class 1) from UOW and is currently a 3rd-year PhD candidate at UOW and IHMRI under Dr. Watson and Associate Professor Sluyter focussing on identifying therapeutic strategies against GVHD. His research interests include identifying genetic biomarkers to predict GVHD and understanding the role of different immune cells in GVHD progression.

Dr. Hong Chee Chew received a Mentee-Mentor Award for his work in Clinical DCD Heart Transplantation under the mentorship of Professor Peter Macdonald and A/Prof Kumud Dhital at St Vincent’s Hospital, Sydney. Their pre-clinical work using porcine model has led to the world’s first distant procurement DCD heart transplantation that was performed in July 2014 in Sydney, Australia. The heart transplant unit has since performed 27 successful DCD heart transplants. The unit pioneered the direct procurement protocol for DCD hearts and the use of normothermic machine perfusion for the assessment and recovery of these hearts.
Dr. Chew received his Masters in Surgery from the University of Sydney. He is currently a PhD candidate at the University of New South Wales, Sydney; and is a cardiothoracic registrar at St Vincent’s Hospital, Sydney. His research focus is on cardiac organ protection strategies including pharmacological and machine technology with the aim to popularise the use of DCD hearts, as well as the development of better assessment tools for the safe utilisation of these organs.

Dr. Jianing Fu was granted an International Transplantation Science Mentee-Mentor Award for her research paper, "Role of Graft-derived Graft-versus-Host T cells in Facilitating Multilineage Blood Chimerism after Human Intestinal Transplantation". Mixed lymphocyte reaction and high throughput TCRβ CDR3 DNA sequencing were used to identify alloreactive T cell clones, and they were further tracked in blood, allograft and bone marrow post-transplant. The data demonstrated that donor GvH-reactive T cells expanding within the graft in response to recipient antigen-presenting cells entering the graft migrate into the recipient circulation and bone marrow, playing a key role in promoting and maintaining mixed chimerism.
Dr. Fu received her BS and MS on Pharmaceutical Sciences and Chemical Biology from Peking University in China, and PhD on Cancer Biology and Immunology from University of South Florida and H. Lee Moffitt Cancer Center. She is now an Associate Research Scientist at the Columbia Center for Translational Immunology at Columbia University. Under Dr. Megan Sykes’s mentorship, Dr. Fu’s research is aimed at decoding the bidirectional alloreactivity after intestinal transplantation and investigating the phenotype and function of gut hematopoietic stem cells and progenitors, with the ultimate goal of eliminating graft rejection by inducing long persistent blood mixed chimerism.

Ahmer Hameed received a Mentee-Mentor award for his work comparing deceased donor liver transplantation outcomes after livers were retrieved using cold in situ perfusion of the donor via the aorta alone, or the aorta and portal vein (“dual” perfusion). A national (Australian) cohort was included over a ten year period. In ‘standard’ criteria donors it was found that overall recipient and graft survival did not significantly differ despite the technique used. However, in a subgroup of patients where livers from higher risk donors were utilized, dual perfusion appeared to provide a significant survival advantage.
Dr Hameed received his medical degree from the University of New South Wales (Australia). He temporarily interrupted his surgical training to undertake a PhD at the University of Sydney under the guidance of Professor Wayne Hawthorne, Professor Henry Pleass, and A/Professor Natasha Rogers, and is currently in the final year of the PhD. The primary focus of his research is the investigation, optimization, and implementation of normothermic machine perfusion prior to kidney transplantation, with a secondary focus on in situ retrieval practices and their subsequent outcomes.

Dr. Melisa Pucci Molineris received a Mentee-Mentor award for her work about acute cellular rejection (ACR) in intestinal transplantation. Mentored by Dr. Gabriel E. Gondolesi, this work evaluates the cellular targets of apoptosis during an ACR event after intestinal transplantation. Although crypt epithelial cell apoptosis is used as histopathological criteria for ACR diagnosis, it was not clear which of epithelial lineages are the target of ACR. Particularly, this research studies integrity and functionality of Paneth and stem cells during different degrees of ACR, in comparison with other clinical conditions such as graft vs. host disease (GVHD) and ischemia reperfusion injury (IRI) that have defined targets.
Dr. Pucci Molineris received her PhD from the Universidad de Buenos Aires in Argentina and this research formed part of her PhD thesis. She is currently a post-doctoral research associate at the Instituto de Investigaciones Bioquímicas de La Plata (INIBIOLP) at the Universidad Nacional de La Plata and works on bacterial urinary tract infections in pregnant women.

Dr. Elisa Montanari received a Mentee-Mentor award for her work about the beneficial effects of human Mesenchymal Stromal Cells (MSC) on porcine hepatocytes. Mentored by Prof. Leo Buhler, this work optimizes and standardizes a protocol for porcine hepatocyte isolation from 10-kilo pigs. To increase pig hepatocyte viability after encapsulation, a novel alginate-PEG-SH grafted hydrogel compared to commercial alginate hydrogel was adopted for hepatocyte encapsulation. This work demonstrated that viability, albumin secretion and diazepam metabolism capacities were maintained in free and alginate-PEG-SH encapsulated hepatocytes. Further, pig hepatocytes co-encapsulated and co-cultured with human MSC showed improved viability and albumin secretion, suggesting that MSC provide a supportive environment.
Dr. Montanari obtained her PhD from the University of Geneva. She is currently a post-doctoral research associate in the Laboratory of Surgical Investigations at the University of Geneva. Her research focuses on optimizing protocols for encapsulation of pig hepatocytes and neonatal porcine islets using newly developed hydrogels, with the aim to establish preclinical studies of pig to human xenotransplantation for the treatment of diabetes and liver diseases.

Dr. Yeung received Mentee-Mentor Award for his study on the significances of M2 macrophages in post-transplant recurrence in hepatocellular carcinoma. Mentored by Prof. Kwan Man, this work identified that liver graft injuries induced the accumulation of M2 macrophages expressed with ΔPD-1. In return, such unique population exhibited increased tumor promoting activities which contributed to poor relapse free survival and tumor recurrence in patients who received liver transplantation. Future studies are planned to target ΔPD-1 M2 macrophages for attenuating the post-operative tumor recurrence incidents and improving clinical outcomes.
Dr. Yeung obtained his bachelor’s and master’s degree in the University of Birmingham and University of Bath, UK. Afterwards, he pursued his PhD in Prof. Man’s laboratory in Department of Surgery, the University of Hong Kong, studying the tumor promoting macrophages in liver cancer. Currently, Dr. Yeung is a post-doctoral fellow under the supervision of Prof. Man and his major research focuses on understanding the roles of macrophages in liver transplantation and cancer for improving diagnostic and therapeutic strategies. In 2016, he received the young investigator award in the same conference. Recently, he also received the rising star and young investigator awards in two conferences International Liver Transplantation Society (ILTS) Annual Congress and Hong Kong Society for Immunology Annual Meeting 2018 on his work on the activation mechanisms of macrophages in transplantation and cancer.


Marina Pérez-Flecha won the Challenging Case Competition for her work on Multivisceral Transplantation immunosuppression. Marina completed her work under the mentorship of Dr. Óscar Caso, Dr Jorge Calvo and Dr Carlos Jiménez, members of the Hepato-Biliary-Pancreatic Surgery and Abdominal Organs Transplantation Unit at the Hospital “12 de Octubre” in Madrid (Spain). The clinical case demonstrated the complex management of immunosuppression in this technically demanding transplants and highlights the various factors involved and therefore the need of a multidisciplinary team and a tailored approach in each patient.
Marina Pérez-Flecha obtained her MD in Medicine in the Complutense University of Madrid and her Surgical residence at the University Hospital “12 de Octubre” in Madrid (Spain). She has attended and participated in many national and international congresses related to Abdominal Organs Transplantation and is at this time developing her PhD concerning Liver Donor Steatosis and studying transplant results depending on the steatosis degree and other possible risk factors.

Bryan Christian Gaza Ilagan won the Challenging Case Competition for his work with a case involving a 50 year old female Filipino admitted in 2017 due to dyspnea following a post-kidney transplantation in 2008 for Chronic Glomerulonephritis. The case developed in unusual ways, as the team struggled to determine the cause of the illness and subsequent unexpected complications.
Dr. Ilagan, MD, FPCP, RPh, earned his Medical degree from the Faculty of Medicine and Surgery of the University of Santo Tomas, Manila, Philippines and Bachelor of Science in Pharmacy degree, also in the same institute. He trained in Internal Medicine at the National Kidney and Transplant Institute (NKTI), Quezon City, Philippines and sub-specialized in Adult Nephrology also in NKTI.

Ulrik Stervbo won the Challenging Case Competition for his work on the application of T-cell receptor sequencing to solve a difficult case of acute rejection versus BKV associated nephropathy in a renal transplant recipient. The work was performed under the supervision of Prof. Nina Babel and Prof. Timm H Westhoff; respectively head and director of the Centre for Translational Medicine at the Marien Hospital Herne – University Hospital of the Ruhr University Bochum, Germany. The T-cell receptor sequencing of blood and biopsy revealed a higher frequency of BKV specific
T-cells in the transplant, compared to donor specific T-cells. This lead to a correction of the immune suppressive regime, which in turn alleviated the acute symptoms. Regular follow-ups show a steady and continuous improvement in graft function.
Ulrik Stervbo holds a bachelor’s degree in physics as well as a master’s degree in computer science and molecular biology from Roskilde University, Denmark. He completed his PhD at the German Rheumatism Research Center Berlin (DRFZ), Germany, where he worked on the repertoire of antigen specific regulatory T-cells. He continues to study the repertoires of the immune T and B-cells and its application in health and disease. Of particular interest is development of experimental and analytical methods
When TTS set out to plan the 27th International Congress in Madrid, Spain under the theme “Outcome Driven”, it was with the objective to offer a congress experience where participants could find relevant content throughout the entire scientific program, thus maximizing the learning and sharing opportunities, and have plenty of time to grow their professional network. We hope that everyone who attended was able to “drive their outcome” and return to work with rich information, new contacts and a sense of refreshed energy and passion for our profession.
The Transplantation Society is looking forward to holding its next international congress in Seoul, Korea in collaboration with the Korean Society of Transplantation (KST). KST, the Korean government and the local industry are fully committed to supporting TTS in delivering a highly successful 28th International Congress of TTS.
The theme “Designed to Empower” will be woven throughout the entire program and logistical planning process in our mission to empower participants to learn, network, volunteer and collaborate at the congress, and then take all of this back home and share it with their colleagues
South Korea has an attractive balance between a long, rich heritage of dynasties and a recently developed modern culture which has led to a series of large sporting events such as the 2002 FIFA World Cup, IAAF World Championships 2011 and the Winter Olympics in 2018.
The Congress Center, COEX, is a state-of-the-art congress venue with several hotels in walking distance, allowing participants to maximize their time at the Congress. Participants can expect modern and easy-to-use transportation from the airport and excellent IT infrastructures everywhere in Seoul.
Since its inception in 1969, the Korean Society for Transplantation (KST) has grown into a society with more than 1,100 members that are representing the fields of clinical and basic research for organ transplantation, cell therapy, islet transplantation, immunobiology, genomics and research for the effective and ethical national healthcare system.
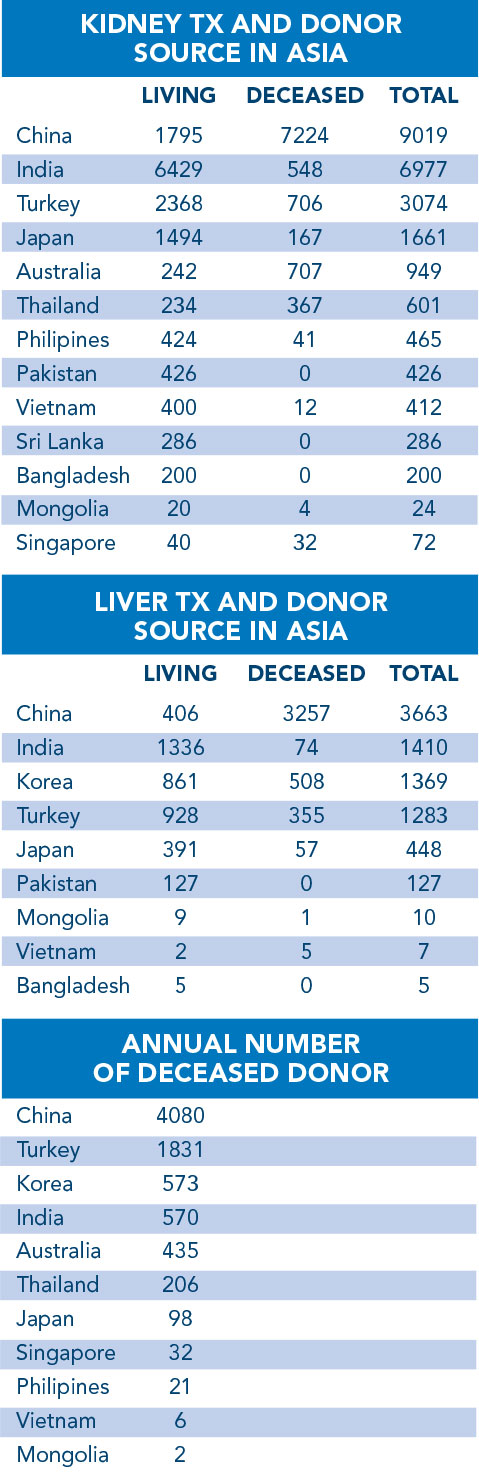
In 2014, a total of 3,300 donations were performed in Korea of which 1,808 were kidney transplants and 1,259 liver transplants. The number of deceased organ donations has increased from 52 in 2000 to 446 in 2014 thanks to the educational programs introduced for organ donation to help change social attitudes. The number of living donor transplantations (kidney, liver) went from 625 in 2000 to 1,855 in 2014.
South Korean doctors performed the first kidney transplant in 1969 and since then have achieved a world class transplantation program. South Korea is on its way to self-sufficiency thanks to the success of the Brain Death Organ Donation Program. With this development, the members of the Korean Society for Transplantation hope to bring this system of ethical transplantation to Central and South East Asia, and thereby contribute to the mission of The Transplantation Society.
Finally, KST is building a “Big Data” registry with the Korea National Institute of Healthcare. About 100 centers and hospitals take part in the KOTRY – Korean Organ Transplantation Registry.
The Program Committee, under the chairmanship of Nancy Ascher and Phil O’Connell, will capitalize on the knowledge and practices found in South Korea.
We invite you to follow our news updates in our weekly Tribune Pulse distributions as well as on the Congress website at www.tts2020.org

The 27th International Congress of The Transplantation Society that was held in Madrid on June 30-July 5, 2018 proved to be a great success. The scientific program offered state-of-the-art information At TTS2018 in Madrid, selected participants of the TTS Leadership series gave presentations on their projects which they developed using the skills and tools they improved upon during the six-month leadership program. These presentations, which concluded the leadership series, will be available for viewing later this Fall – watch for announcements in the weekly Tribune Pulse.
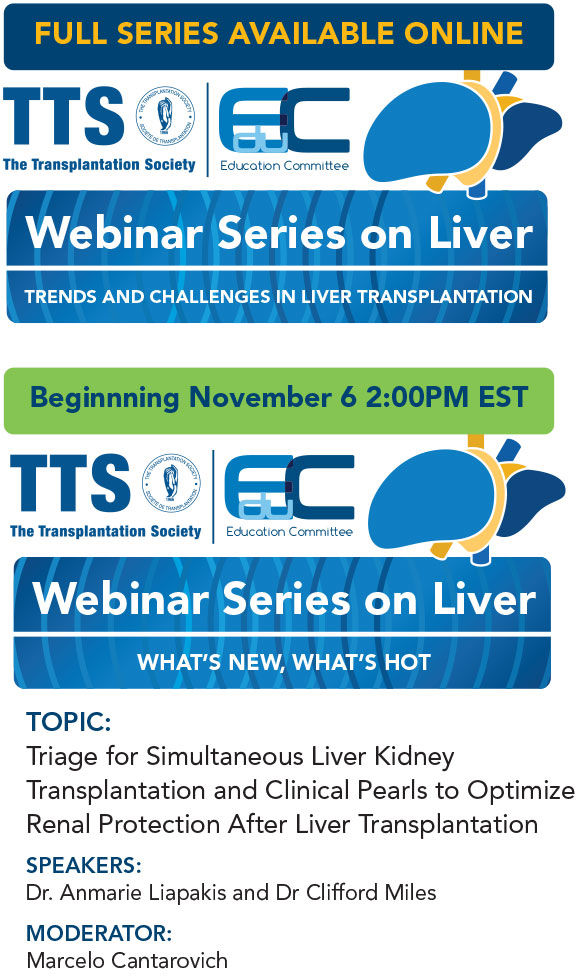
The Education Committee is excited to welcome its new committee members and thank past committee members for their work on the committee over the past two-year term. The Education Committee will be launching the second part of the Liver Webinar Series “What’s New, What’s Hot” this fall and is working on a Precision Transplantation Medicine Webinar series which will be launched in 2019. Other programming is continuously being developed.
TTS Education Committee Co-Chairs
- Medhat Askar
- Marcelo Cantarovich
- Sukru Emre
TTS Education Committee
Members 2018-2020
- Enver Akalin
- Abdulla Al-Sayyari
- Hatem Amer
- Karen Dwyer
- Shannon Grappe
- James Gilbert
- Gabriel Gondolesi
- Maria Kaisar
- Budde Klemens
- Nithya Krishnan
- Kaori Kuramitsu
- Vivek Kute
- Annmarie Liapakis
- Valaria Mas
- Marwan Masri
- Sreelatha Melemadathil
- Fatima Oeyen
- Ruth Sapir-Pichhadze
- Jessica Pinto
- Marlies Reinders
- Manuel Rodriguez-Davalos
- Milagros Samaniego
- Manisha Sahay
- Pablo Uva
- Nam-Joon Yi

Transplantation science formed an integral part of TTS Madrid 2018, which was a hugely successful and enjoyable congress. The Transplantation Science Committee (TSC) contributed to many elements, including the organisation of the Virtual Global Transplantation Laboratory (vGTL) pre-meeting where speakers provided some of the most valuable insights in immune monitoring. These included advice on analysis, new technologies, biobanking, standardization, as well as how to integrate monitoring in advanced clinical trials. The Post-Graduate Course integrated talks from some of the most prominent scientists in transplantation science providing distilled wisdom to members of TTS. A basic and translational science stream was embedded throughout the Congress, demonstrating the importance and impact transplantation science continues to have on the larger field.

The TSC is also delighted to announce the winners of the prestigious Leslie Brent and Anthony P. Monaco Awards for 2018. These two awards are presented to the authors of the two most influential papers published in Transplantation in 2017. The Leslie Brent Award, in recognition of the best basic science paper, is presented to Drs. Duran-Struuck and Sondermeijer for their paper “Effect of Ex Vivo-Expanded Recipient Regulatory T Cells on Hematopoietic Chimerism and Kidney Allograft Tolerance Across MHC Barriers in Cynomolgus Macaques”. The Anthony P. Monaco Award, in recognition of the best translational science paper, is awarded to Dr. Martens for his paper “Humoral Reactivity of Renal Transplant-Waitlisted Patients to Cells from GGTA1/CMAH/B4GalNT2, and SLA Class I Knockout Pigs”.
Here are a few facts
- Transplantation Direct is an official journal of the TTS, and has a close association with our flagship journal Transplantation.
- Being an open access peer-reviewed journal, anyone can disseminate and reuse articles, and reuse the scientific material, provided attribution to the original authors and original source is given. Authors retain ownership of the copyright.
- A wide variety of articles have been published in the field, including content in 22 transplantation-relevant subjects from basic science, to ethics, to the various clinical areas of clinical transplantation.
- We have established an extensive yet streamlined review process, providing first decisions on average within 2.5 weeks.
- Besides conventional articles, the journal serves as a resource for publication of clinical trial protocols of special high interest, new methodologies and registry data.
- The editorial office strongly supports social media dissemination, and promotes video presentation of published articles, increasing overall exposure to the transplantation community.

The YMC is proud to represent the future of transplantation, and to support TTS’ youngest members in their pursuit of careers in transplantation. We have taken our charge from Immediate Past-President Ascher and President Haberal seriously and are most appreciative of the support we receive from them and the TTS Council.
YMC presence at the 27th Congress in Madrid, Spain this past summer was historic. We maintained a presence in the exhibit hall throughout the meeting in the form of the Young Members Corner, which served as a meeting space for our members to connect and collaborate. We held morning symposia focused on career development that featured giants in our field including Drs. Nancy Ascher, Barbara Murphey, and Jeremy Chapman. We also made time to party hosting our second Young Members Night Out! Knowledge Opinion Leaders (KOLs) joined us for the event. It was a great, relaxed evening affording ample opportunities for our young members to connect with new mentors and colleagues from around the globe. We are excited to be a part of TTS and to continue to move the mission of TTS forward. The upcoming year will be filled with additional opportunities for young members to engage within TTS as we aim to develop a webinar series on career development for 2019. Stay tuned for more great things coming from the YMC of the TTS.
In case you missed it:
The Young Members Committee continues to grow with international representation at the trainee and junior faculty levels. Our mission focuses on promoting career development opportunities for our youngest members. This past year has been extremely productive:
-
Consistent presence in the Tribune Pulse with a quarterly article focused on young member related topics as well as spearheading TTS sponsored webinars.
-
The YMC has also connected individuals and institutions. The latest example is the sharing of information and development of critical transplant programs both at the University of Alabama at Birmingham and the University of Cape Town.
You can find all our contributions to the Tribune Pulse on the TTS website at www.tts.org/newstts-world/tribune-pulse-past-issues.


Since the Declaration of Istanbul (DoI) on organ trafficking and transplant tourism was developed in 2008, it had profoundly impacted national legislation of many countries and professional codes of practice. To ensure that the DoI remains a valuable source of ethical guidance for health professionals and policy makers for the next decade in the face of persisting and emerging challenges in organ trafficking and transplant tourism around the world, the Declaration of Istanbul Custodian Group (DICG) updated the original DoI. After a global public consultation drive on the proposed Update, comprehensive feedback was received from our DICG members, members of the two parent organizations – TTS and ISN –, members of organizations that have endorsed the Declaration of Istanbul, and members of the broader transplant community.
The final 2018 version of the DoI was presented on the 1st of July 2018 at 10th Anniversary of the Declaration of Istanbul, as part of the Pre-Congress program at the International Congress of The Transplantation Society. You can find more information about the update process and the final text in different languages at: www.declarationofistanbul.org/about-the-declaration/structure-and-content
The updated DOI, simplified, clarified and improved, includes a preamble, 5 definitions (aligned with those provided by the relevant international legally binding texts) and 11 principles. An explanatory commentary is being developed to help interpret the principles and make proposals for its application in practice.
The 2018 version of the DoI represents an opportunity to renew the commitment of the international transplant community to combat unethical practices that violate fundamental principles and rights and becomes a mandatory reference document for transplant professionals. Please share the 2018 version DoI with your transplant colleagues and community.


The Women in Transplantation initiative of The Transplantation Society has stayed busy over the summer and though the fall.
New Leadership
Steering Committee:
We would like to thank Dr. Elaine Reed whose dedication and leadership has been instrumental in the success of Women in Transplantation. Dr. Reed’s term as Chair ended in July. She has since passed the torch to Dr. Lori West who was Co-Chair for the past two-year term (WIT leadership terms are for two years). Dr. Roslyn Mannon has stepped up from her role on the WIT steering committee to become the WIT Co-Chair.
Pillar 1 – Advancing and Inspiring Women Transplant Professionals:
Dr. Lorna Marson has stepped down from her role as Pillar 1 Chair to focus on her responsibilities as the British Society of Transplantation’s President. We welcome Christine Falk as the new Chair of Pillar 1. We thank her for her dedication and service.
Pillar 2 – Championing Issues of Sex and Gender in Transplantation:
We are happy to announce that Dr. Bethany Foster will continue in her role as Chair of Pillar 2.


WIT at TTS 2018
WIT hosted a pre-meeting session at TTS2018 in Madrid on Saturday, June 30th entitled “Advancing Women in Transplantation through Leadership and Advocacy of Issues Related to Sex and Gender.” Two sessions are available online: Increasing Leadership of Women in Transplantation: Challenges and Solutions with presentations from Dr. Lori J. West and Lisa A. Robinson as well as Creating a Culture of success & steps to improve equity with presentations from Dr. Maria Irene Bellini, Dr. Vassilios Papalois and Dr. Deborah Adey. You can view these presentations by going to https://tts.guide/webapp/programme/1
Congratulations:
WIT would like to congratulate two influential WIT leaders: WIT founder and current Advisory Board Member Kathryn Wood as well as WIT Steering Committee Member Megan Sykes on winning their Medawar Prizes (see page 11 for details).
Recent Networking Events:
WIT held two events this summer. The first was at the American Transplantation Congress in Seattle which was held jointly with AST’s Women’s Health Community of Practice (WHCOP). This event was sponsored by Novartis and Veloxis. Dr. Diane McKay, the incoming president of the American Society of Transplantation discussed the barriers and successes of academic careers for women. We appreciate the support of Novartis, Veloxis and One Lambda for these events.
 On October 4th, WIT held a networking breakfast at the American Society for Histocompatibility and Immuno-genetics where Dr. Hannah Valentine, NIH’s first Chief Officer for Scientific Workforce Diversity described NIH’s current approach and activities related to promoting inclusive excellence through fostering workforce diversity. This event was sponsored by One Lambda, Inc.
On October 4th, WIT held a networking breakfast at the American Society for Histocompatibility and Immuno-genetics where Dr. Hannah Valentine, NIH’s first Chief Officer for Scientific Workforce Diversity described NIH’s current approach and activities related to promoting inclusive excellence through fostering workforce diversity. This event was sponsored by One Lambda, Inc.
Upcoming Networking Events:
A networking dinner is being planned in Chicago on November 15th at SUNDA restaurant. The dinner will provide an intimate setting for transplantation professionals in the Chicago area to network and discuss mutual issues and achievements. For more information about this event as well as to learn about how you can host a local WIT event in your city contact: wit@tts.org.
To consolidate its position as the leading global organization, the Council has representation from all six regions of the world: Asia, Europe, Latin America, Middle East/Africa, North America and Oceania.
EXECUTIVE MEMBERS
| PRESIDENT Mehmet Haberal |
| PRESIDENT-ELECT Marcelo Cantarovich |
| IMMEDIATE PAST PRESIDENT Nancy Ascher |
| VICE-PRESIDENT Elmi Muller |
| SECRETARY John J. Fung |
| SENIOR TREASURER Stefan G. Tullius |
| TREASURER Minnie Sarwal |
| HISTORIAN Randall E. Morris |
| INTERNATIONAL HEADQUARTERS Jean-Pierre Mongeau Executive Director |
COUNCILORS
| ASIA Hiroto Egawa S. Adibul Hasan Rizvi |
| EUROPE Peter J. Friend Martí Manyalich Vidal |
| LATIN AMERICA Maria Gerbase De Lima Gabriel E. Gondolesi |
| MIDDLE EAST / AFRICA Mustafa Al-Mousawi |
| NORTH AMERICA Medhat Askar Peter G. Stock Megan Sykes |
| OCEANIA Karen Dwyer Steven J. Chadban |
The deadline to submit an application is JANUARY 1, 2019
The TTS-ILTS Paired Transplant Centers Program is a collaboration between The Transplantation Society (TTS) and the International Liver Transplantation Society (ILTS) supporting new liver transplant programs in emerging countries.
TTS-ILTS program advertisement and question request: Please submit any questions you have about the program or the application process to initiatives@tts.org.
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!