Fourth International Consensus Guidelines on the Management of Cytomegalovirus in Solid Organ Transplantation
We are excited to announce the publication of the Guidelines in our journal Transplantation.
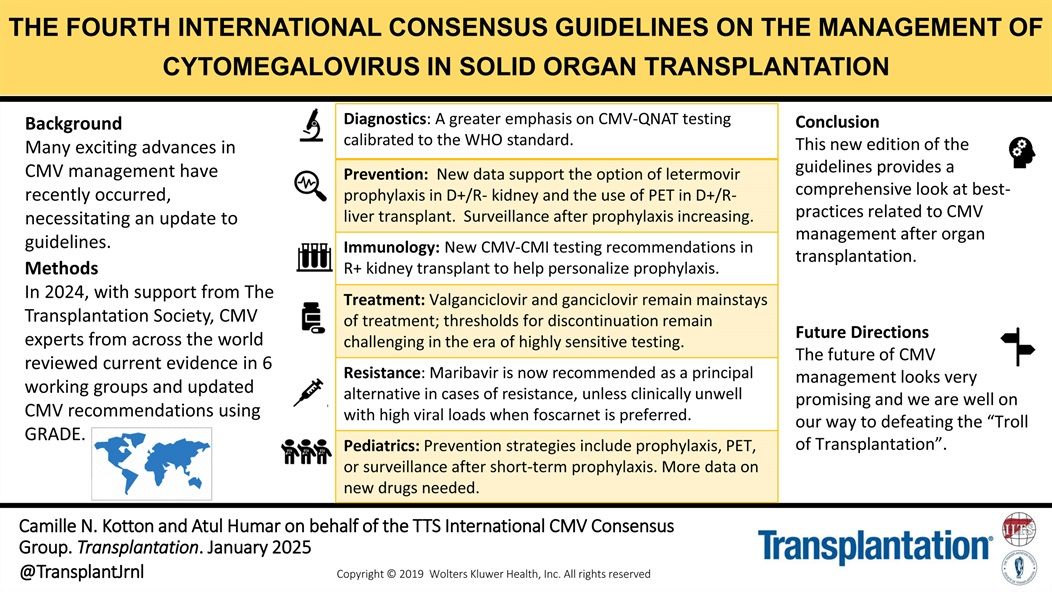
This updated international consensus—developed by leading CMV experts with support from The Transplantation Society—reflects major advances in CMV diagnostics, prevention, treatment, and surveillance.
These guidelines offer a comprehensive framework for optimizing CMV management post-transplant and chart a clear path toward overcoming what is often called the “Troll of Transplantation.”
Highlights include:
- Emphasis on CMV-QNAT testing aligned with WHO standards
- Expanded role for letermovir prophylaxis and PET in D+/R– transplants
- New immunologic testing to personalize prophylaxis in R+ kidney recipients
- Updated guidance on valganciclovir, ganciclovir, and maribavir use
- Pediatric strategies and the need for novel drug development
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!
