May 21, 2025
Xenotransplantation:
Updates from the Transplantation Journal
Video Replay
Local time (Corresponding local time at your current location)
Overview
Outline
International Xenotransplantation Association (IXA) Position Paper on the History, Current Status, and Regulation of Xenotransplantation
- How xenotransplant research started and what are the major barriers to success.
- Research and innovation – haem & immunological barriers, genetic engineering etc.
International Xenotransplantation Association (IXA) Position Paper on Kidney Xenotransplantation
- Key clinical trials in xenotransplantation.
- Issues with rejection and infection.
- Promise and what are the questions still to be answered moving forward.
International Xenotransplantation Association (IXA) Position Paper on Infectious Disease Considerations in Xenotransplantation
- What are the major zoonotic infections which have been encountered so far?
- What challenges exist in diagnosis/treatment of these?
- What are the current tools and research pipelines to help address this problem?
Optimizing IVIg in Xenotransplantation: A Call to Action
- What role does IVIG have in xenotransplantation?
- What is the concern about using IVIG and what can be done to address these?
The International Human Xenotransplantation Inventory: Current Data and Future Directions
- How can global efforts be best focused to moving the field forward?
- Discussion around key aspects of a xenotransplantation inventory and data harmonisation.
- Development of guidance, regulatory oversight and ethics in the field.

Organizer
Bio
Nephrologist, Westmead Hospital Clinician Researcher Westmead Institute for Medical Research
Jen is a transplant nephrologist working at Westmead Hospital and has an active research portfolio in cell therapies and transplant genomics at the Westmead Institute for Medical Research.

Chair
Bio
Professor of Surgery
Transplant Surgery
University of Maryland
I have been involved continuously in basic cellular and molecular transplant immunology for 30 years. My basic research has always focused on T cell immunobiology, and for more than 20 years has also focused on issues of migration, trafficking, secondary lymphoid organ structure and function, and lymphatic structure and function, and how these processes and structures influence T cell immunity and T cell tolerance in models of transplantation. I have also maintained an active clinical practice in solid organ transplantation and am thus constantly exposed to the problems of patients and their immune systems, including cellular and humoral rejection, opportunistic infections, chronic viral disease, autoimmune organ failure, and immunosuppression medication side effects. I have had over 60 trainees in my lab, am actively involved in teaching and mentoring, and have served on T32 faculty.

Chair
Bio
Professor of Surgery and Director of the Cardiac Xenotransplantation Program
University of Maryland School of Medicine (UMSOM), Baltimore, MD.
Dr Mohiuddin previously served as Chief of Transplantation of the Cardiothoracic Surgery Research Program at the National Heart Lung and Blood Institute / NIH. He held faculty positions at the University of Pennsylvania, Philadelphia, and Rush University, Chicago. He has been involved in Xenotransplantation Research since 1991 and started xenotransplantation research programs at the NIH, Rush University Chicago, and UMSOM.
He is Associate Editor for Transplantation and Frontiers in Transplantation. He reviews manuscripts for many other journals. He is also the past president Dow Graduate Association of North America. His achievements include several NIH and non-NIH grants during his academic career. He is a member of the executive committee of COTS and a member of the advisory council of TRM of the AST. Dr. Mohiuddin's career has been a testament to his pioneering spirit in Xenotransplantation. His groundbreaking contributions include demonstrating the most prolonged survival in heterotopic and orthotopic cardiac Xenotransplantation. He devised an immunosuppression regimen that significantly extended the lifespan of cardiac xenografts to nearly three years. His leadership extends to IXA, where he now serves as President, following four years as Councilor and a liaison to foster productive dialogue between the IXA and the FDA. His extensive publication record includes over 120 papers and more than 150 abstracts, and he has been a sought-after expert speaker on Xenotransplantation on numerous occasions.
Dr. Mohiuddin's unwavering dedication to Xenotransplantation culminated in two significant milestones. On January 7, 2022, he achieved the First Genetically Engineered pig-to-human cardiac Xenotransplantation, a feat that marked a new era in the field. He then led the second xenotransplantation procedure in September 2023, further solidifying his position as a leader in the field. Both surgeries were successfully conducted at the University of Maryland Medical Center in Baltimore, Maryland. Besides other awards, Dr Mohiuddin was picked by Nature Journal as one of the “Nature’s 10” in 2022. Ten people who helped shape science in 2022.

Speaker
Bio
Professor of Transplantation at the Department of Surgery
The University of Sydney
Director of the National Pancreas and Islet Transplant Laboratories,
Westmead Hospital, Westmead, New South Wales, Australia.
Dr. Wayne Hawthorne is the Immediate Past President of IXA, the current of ACBMS, Chairman of the Xenotransplantation Working Group of TSANZ and Chairman of WSLHD AEC. Professor Hawthorne’s career has focused on treatments for Type 1 Diabetes (T1D), having established the laboratory and techniques to perform islet cell isolation for Australia's First Clinical Islet Transplant program. Paralleling the clinical program Professor Hawthorne has dedicated his research career to establishing Australia’s Islet Cell and Xenotransplantation research programs, obtaining ongoing NH&MRC and Breakthrough T1D grants for more than 25 years. He developed and has established the Westmead NHP facility and has extensive small and large animal surgical and anaesthetic expertise that few others have in Australia. He has decades of expertise in large animal models, including the use of NHP’s in research. He continues to oversee the islet and xenotransplant research programs at the Westmead facility and remains dedicated to continuing to develop and provide the most Ethical and humane ways to run medical research projects and ensure their translation to the clinic along with the training of many scientists, clinicians and surgeons.
A founding researcher of the Centre for Transplant and Renal Research at the Westmead Institute for Medical Research and The University of Sydney, he remains responsible for surgical research projects where he has supervised many students including; PhD, Masters, Hons and MD students. He continues to build on the success of his research work and his team have translated the transplantation treatments for T1D, being the first in Australia to perform both Clinical Allo and Auto islet cell isolation and transplantation and to perform islet after kidney and combined islet/kidney transplants into clinical patients. He has performed more than 330 clinical islet isolations and islet cell transplants in both adults and children as young as 4 years of age. He has >250 journal publications, 20 books and book chapters and >700 published proceedings along with presenting numerous International and National presentrations.

Speaker
Bio
Associate Professor of Surgery
University of Maryland, Baltimore, MD, USA
Raphael P. H. Meier, M.D., Ph.D. is a liver, kidney, and pancreas transplant and hepatobiliary surgeon and Associate Professor of Surgery at the University of Maryland School of Medicine. Dr. Meier completed his medical education at the University of Geneva School of Medicine in Switzerland, followed by a general surgery residency at the University of Geneva Hospital. He further honed his expertise through a fellowship in abdominal transplant surgery at the University of California, San Francisco (UCSF).
His research spans both basic science and clinical aspects of abdominal organ transplantation. Dr. Meier has made significant contributions to the field, developing microencapsulated and genetically modified mesenchymal stem cells to deliver anti-inflammatory cytokines for treating chronic liver diseases. His work also explores the genetic factors influencing islets of Langerhans function and their impact on diabetes and islet transplantation outcomes. Currently, Dr. Meier is part of a conducting effort to develop suitable xenogeneic organs, addressing the critical worldwide organ shortage.
A prolific researcher, Dr. Meier has authored over 100 scientific manuscripts and book chapters, published in prestigious journals including JAMA Medicine, Journal of Hepatology, American Journal of Transplantation, and Transplantation.
Dr. Meier is actively involved in several international organizations. He serves as a councilor of the International Xenotransplantation Association and is a committee member of the Basic and Translational Research Committee for the International Liver Transplantation Society. Additionally, he is a member of the American Society of Transplant Surgeons and the American Society of Transplantation, further demonstrating his commitment to advancing the field of transplantation medicine.

Speaker
Bio
Professor of Medicine, Harvard Medical School
Director, Transplant Infectious Disease and Compromised Host Service
Associate Director, Transplant Center, Massachusetts General Hospital
Jay A. Fishman, M.D., FACP, FAST, FIDSA is Professor of Medicine at Harvard Medical School, Director of the Transplant Infectious Diseases Program at the Massachusetts General Hospital (MGH), and Associate Director of the MGH Transplant Center. Dr. Fishman completed medical school at Johns Hopkins University School of Medicine, internal medicine training and Infectious Disease Fellowship at MGH, and Fellowships in Molecular Biology and Genetics at MGH and Harvard Medical School. Dr. Fishman established the Transplant and Immunocompromised Host Program of the MGH, the first such training program worldwide which has trained many leaders in this field. He established Transplant Infectious Disease as an essential component of Transplant programs. His clinical expertise spans infectious diseases and immunology of solid organ and stem cell transplant recipients and has defined approaches to managing infectious risk in allo- and xenotransplantation. His research defined the use of ganciclovir for cytomegalovirus infection in transplant recipients. His background in immunology, virology and molecular biology led to the cloning of surface antigens of human Pneumocystis, and to studies of porcine cytomegalovirus and porcine endogenous retrovirus (PERV) in xenotransplantation. He has over 300 peer-reviewed publications and is internationally recognized as a clinician-educator and scientist. He is Past-President of the American Society of Transplantation and is President-elect of the International Xenotransplantation Association. He has received career achievement awards from AST and TTS.

Speaker
Bio
Professor of Medicine, Division of Infectious Diseases
Johns Hopkins University, Baltimore, MD, USA
Robin Avery MD, FIDSA, FAST is Professor of Medicine in the Division of Infectious Diseases, and Education Director for the Transplant/Oncology ID Program at Johns Hopkins. Earlier in her 32-year career, she was the founding Section Head of Transplant ID at Cleveland Clinic. She is a past chair of the American Society of Transplantation (AST) Infectious Disease Community of Practice, and has served on many guidelines committees for transplant and ID societies. Her areas of interest include transplant-related viral infections, novel therapies for CMV, post-transplant hypogammaglobulinemia, COVID therapeutics, strategies for safer living after transplantation, and most recently, prevention and treatment of infections in xenotransplantation. She was awarded the 2017 Best Consulting Physician Award at Johns Hopkins, the 2022 AST ID Community of Practice Robert Rubin Award, and the 2023 AST Mentorship Award.

Speaker
Bio
Professor, University of Fribourg
Fribourg, Switzerland
President of the International Xenotransplantation Association (2017-2019)
Use the image below to promote this event (right-click to download)
Xenotransplantation White Papers in Transplantation
Hawthorne, Wayne et al.
Transplantation ():10.1097/TP.0000000000005373, April 08, 2025. | DOI: 10.1097/TP.0000000000005373
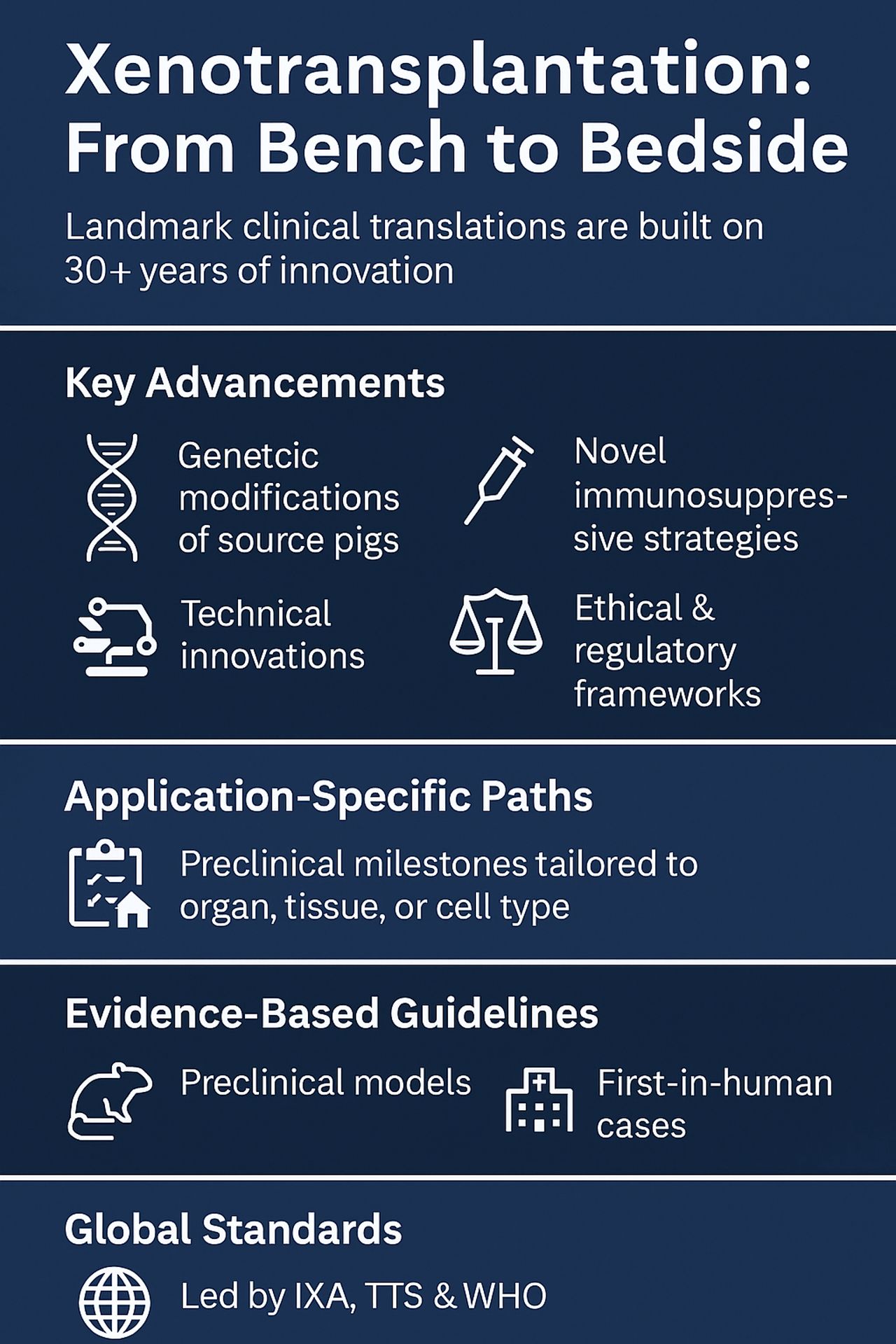
Landmark xenotransplants are the result of 30+ years of innovation—genetic, immunologic, ethical & regulatory. The IXA, TTS & WHO have laid the groundwork for safe clinical translation.
Key breakthroughs include:
- Genetic modifications of source pigs
- Novel immunosuppressive strategies
- Technical innovations
- Development of ethical & regulatory frameworks
Hu, Xiaowei et al.
Transplantation ():10.1097/TP.0000000000005367, April 08, 2025. | DOI: 10.1097/TP.0000000000005367
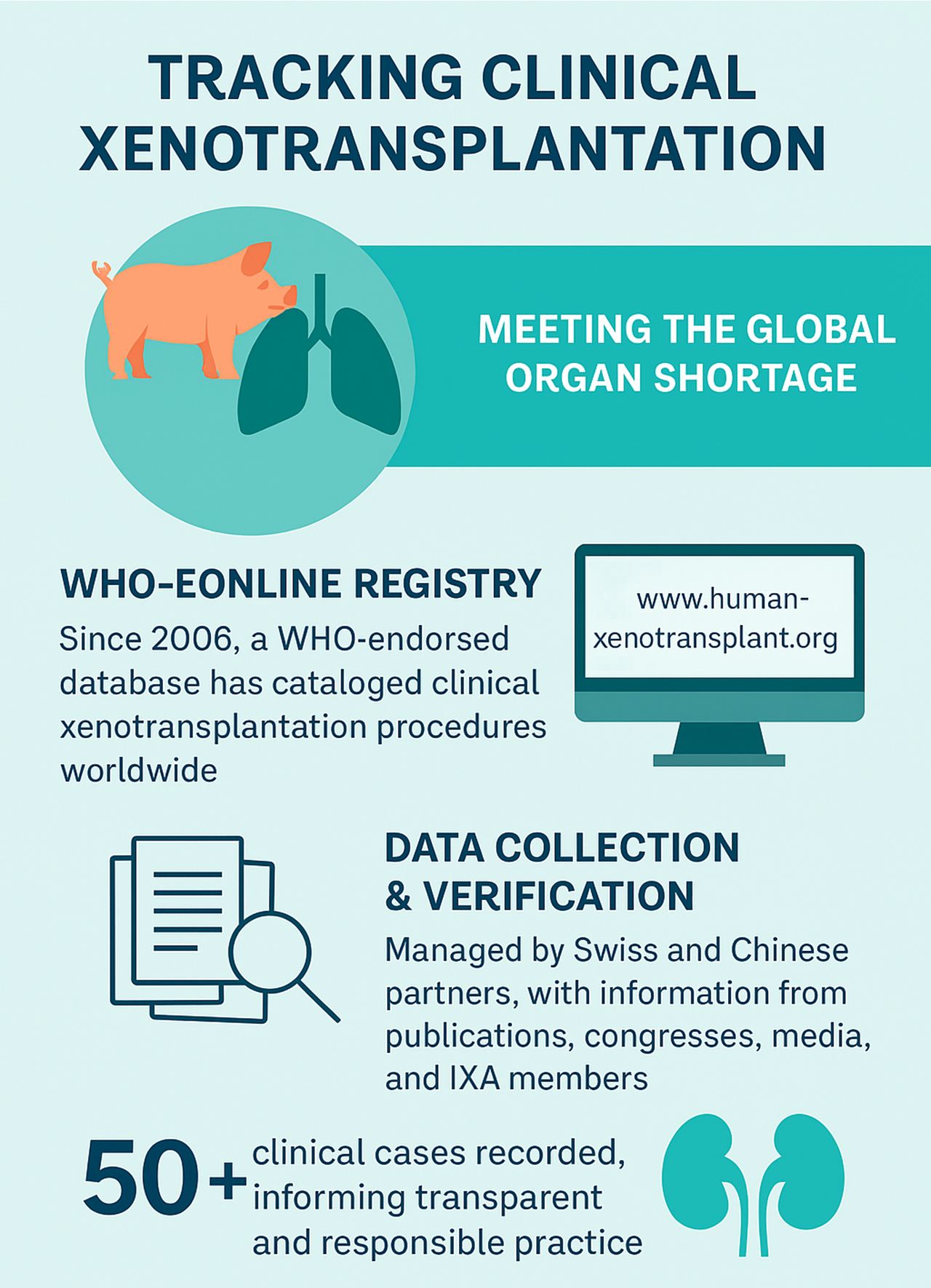
Xenotransplantation is emerging as a solution to the global organ shortage. Since 2006, a WHO-endorsed registry has tracked 50+ clinical cases—paving the way for safer, transparent progress.
Over the past 2 decades, the registry reveals key trends:
- Types of xenotransplants
- Source animals (mostly pigs)
- Regulatory status of procedures
- Countries involved
Tector, A. Joseph
Transplantation 109(2):p 231-234, February 2025. | DOI: 10.1097/TP.0000000000005223
Thirty years after the initial strategies to develop genetically engineered pigs for use as organ donors in xenotransplantation were described, evaluation of these pig organs in humans has begun.
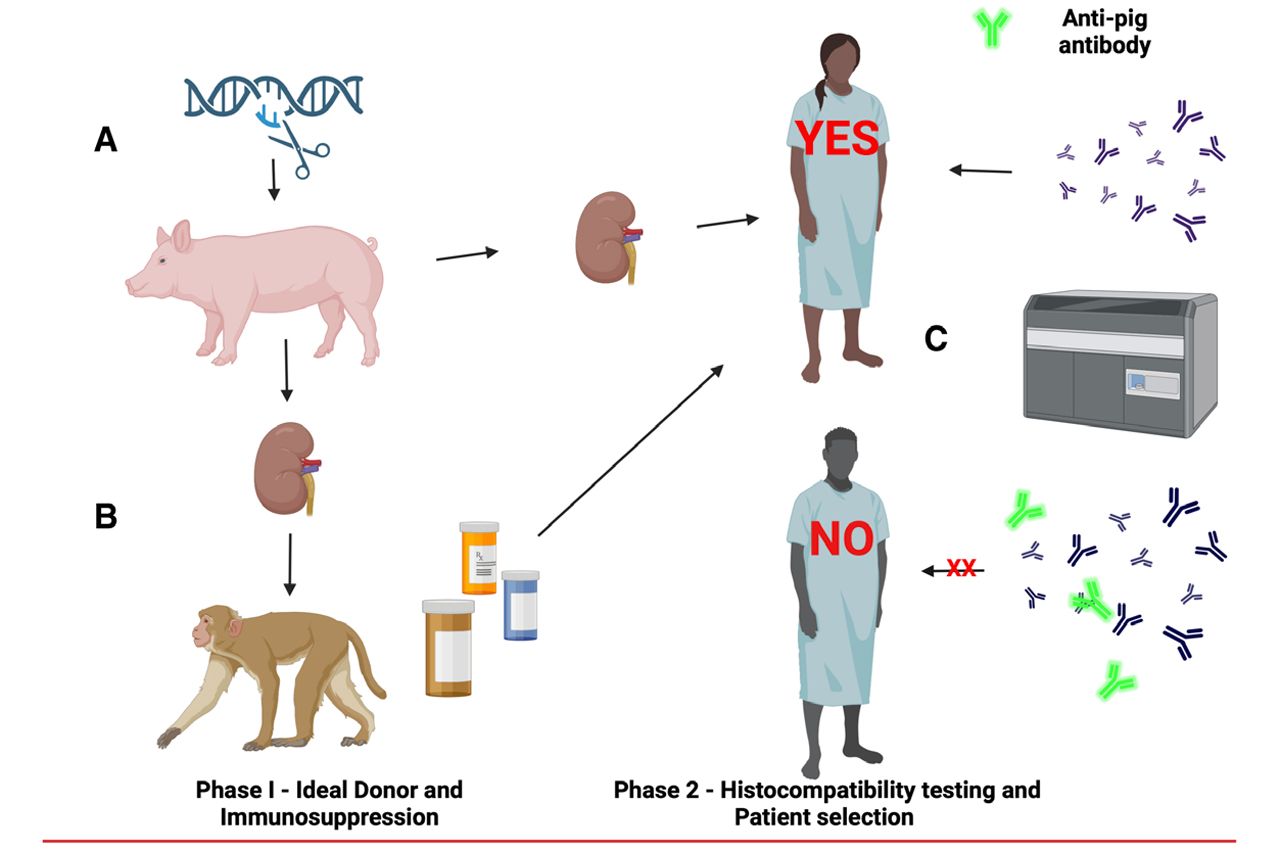
Special Feature:
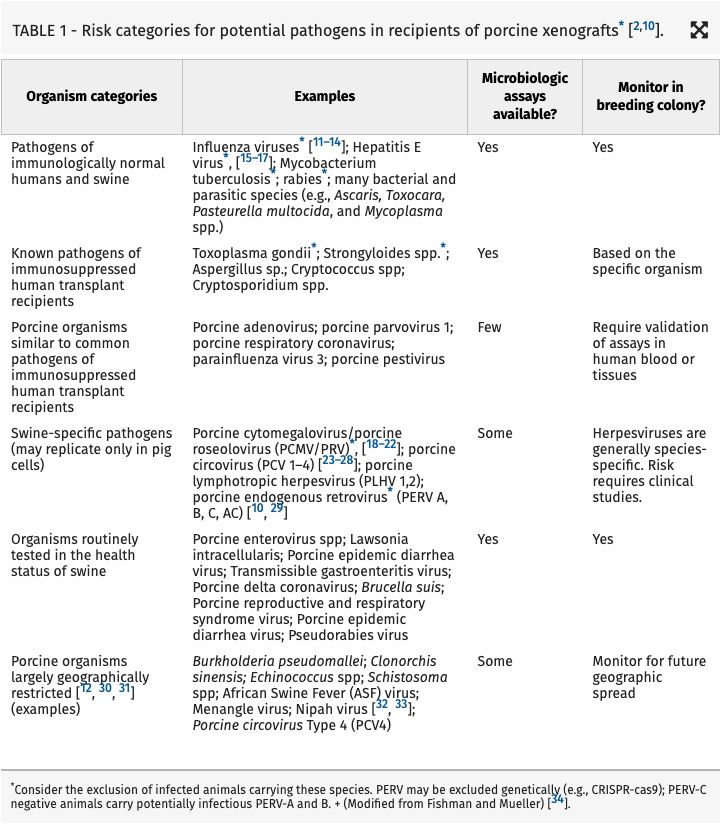
Fishman, J. A., Denner, J., & Scobie, L. (2025).
Transplantation ():10.1097/TP.0000000000005371, April 08, 2025. | DOI: 10.1097/TP.0000000000005371
Related Links
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!