Have you used the Clinical Decision Support Tool (CDST)?
Cytomegalovirus (CMV) is a formidable pathogen that can cause significant morbidity and mortality in patients who undergo lifesaving solid organ transplant (SOT) or hematopoietic cell transplant (HCT). Traditional antivirals, including ganciclovir, valganciclovir, foscarnet, and cidofovir have long been the mainstays for CMV treatment, although they are not FDA approved for treating CMV infection or disease in the post-transplant setting. Side effects, including hematologic and renal toxicity, limit the use of traditional antivirals.
Prior to FDA approval in 2021 of a new antiviral, maribavir, there were no FDA approved therapies for drug-resistant or refractory CMV in SOT or HCT patients. Maribavir is an important advancement in CMV therapy since it does not cause hematologic or renal toxicity and it is an oral medication.
In order to assist clinicians with evaluation and treatment selection for resistant or refractory CMV in their SOT or HCT patients, an international panel of CMV experts developed a novel web-based point-of-care tool called the Resistant/Refractory CMV in Transplantation Clinical Decision Support Tool (CDST). The CDST is accessible free of charge from any desktop, laptop, or mobile device at www.CDSTforCMV.com. The new antiviral maribavir is incorporated into the CDST treatment algorithms.
Transplantation Updates

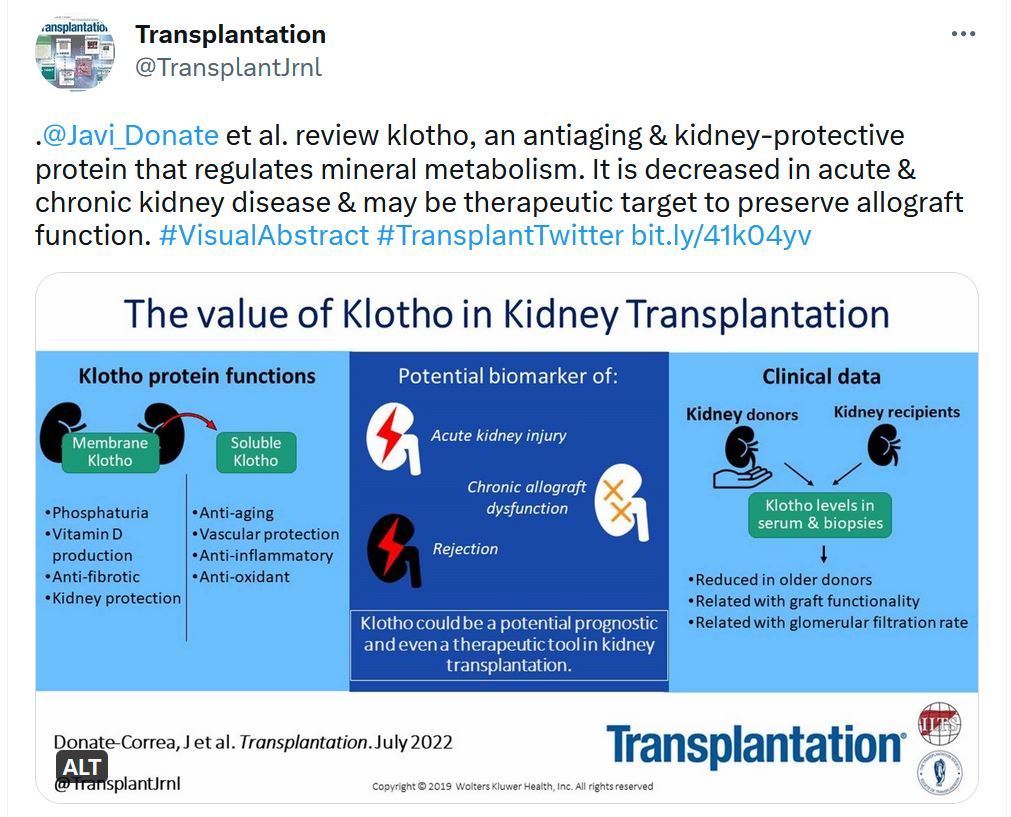
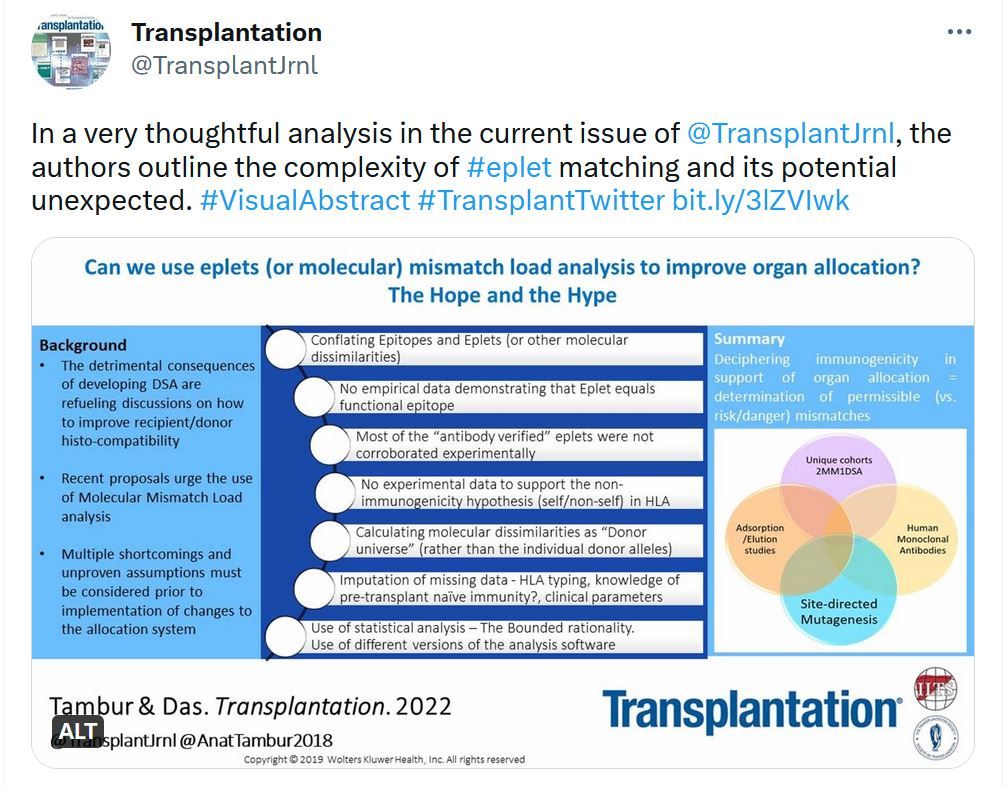
Transplantation - Highlighted Tweets
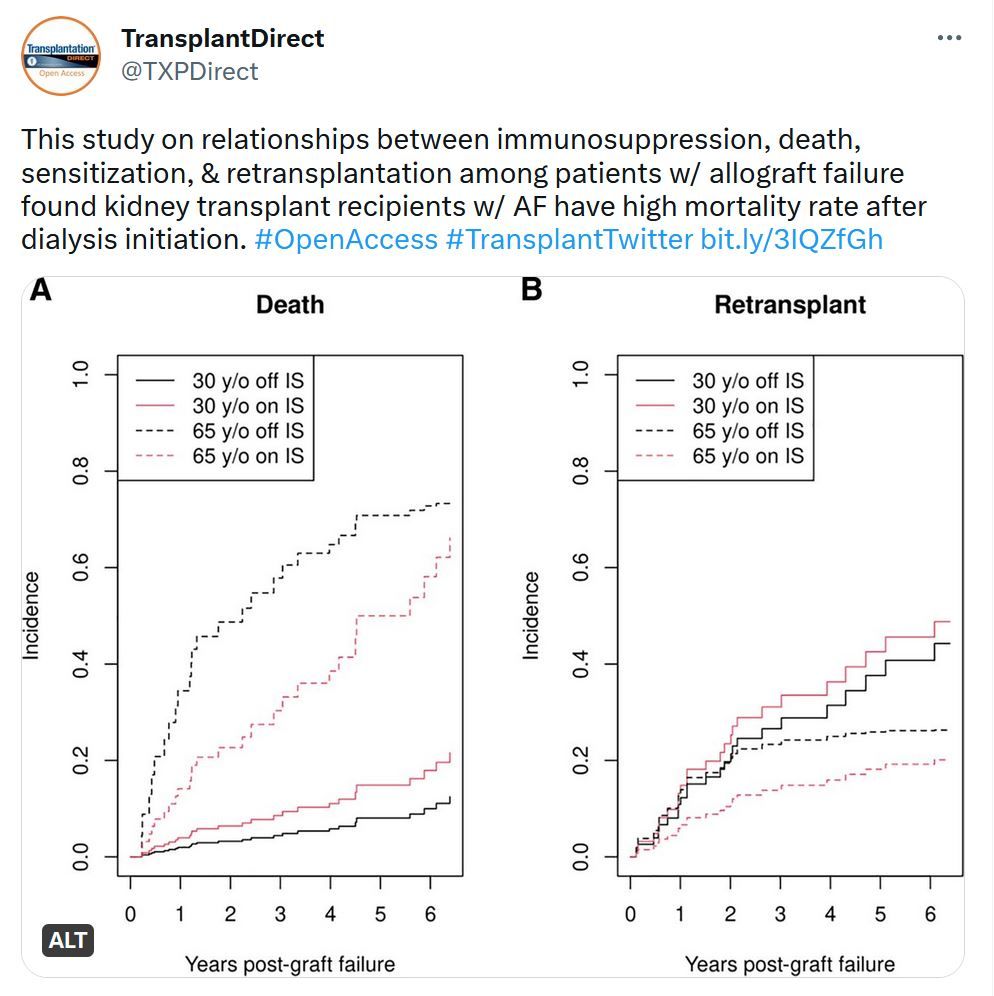
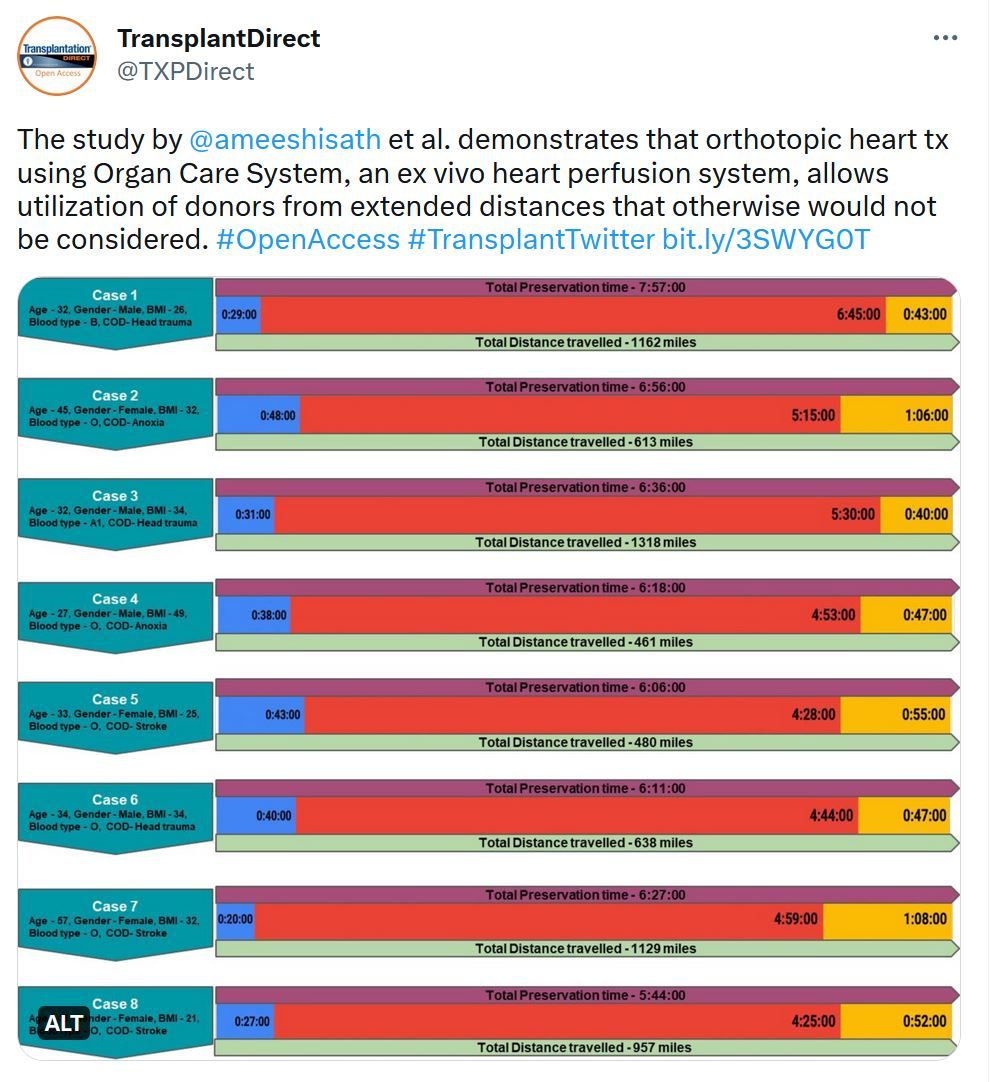
Transplantation Direct - Highlighted Tweet
Survey on Transplant Infections
Australasian Kidney Trials Network
Identifying patient, caregiver and health professional perspectives for the design of a trial to prevent urinary tract infection in kidney transplant recipients
Deadline - March 31, 2023


Urinary tract infections survey - invitation to participate in a 5-10 minute survey to identify important aspects to include in a clinical trial.
Urinary tract infection after transplantation was identified by patients, caregivers and health professionals as an important infection.
We are inviting patients, caregivers and health professionals to complete a survey to identify what aspects of urinary tract infections are important, when designing a clinical trial to prevent transplant-associated urinary tract infections. These aspects may include the population to target, the treatments to evaluate, and the outcomes that are important to report in the trial. Participation is voluntary and anonymous, and you may exit the survey at any time.
Further information can be found on the Participant Information Sheet. All participants will receive a copy of the results. The survey will close March 31, 2023.
Please complete these questions on your own. By clicking “begin”, you are consenting for your data to be used for research purposes.
Click the following link to begin: Start survey or copy and paste the URL (https://uqmedicine.syd1.qualtrics.com/jfe/form/SV_d42Jdb9JDXvpG6i) into your Internet browser:
Your opinion will be valuable and important to help ensure that we consider the most important aspects of urinary tract infection in kidney transplant trials. Participation in this survey will not impact the care provided to patients in any way. If you have any questions, please email contact us at samuel.chan@uqconnect.edu.au
If you would like to speak to an officer of the University not involved in the study, you may contact the Ethics Coordinator at 617 3365 3924 / 617 3443 1656 or email (humanethics@research.uq.edu.au)
Thank you for considering the invitation to participate.
Research Grants Program

Women in Transplantation Research Grants

2023-2025 WIT Fellowship Grants and
Inaugural LMIC Grants
for Research in Gender and Sex in Transplantation
Report on earthquake that hit Turkey and Syria

Upcoming Meeting Announcements
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!