CORONAVIRUS (COVID-19) UPDATE DASHBOARD
The Transplantation Society (TTS) and our journal Transplantation have developed online resources to keep you informed on the Coronavirus (COVID-19) outbreak.
- TTS Coronavirus (COVID-19) Dashboard
www.tts.org/covid-19 - Transplantation Global Transplantation COVID Report
www.tts.org/txjcovid19
We are also requesting contributions and news from the transplant community to be sent to covid-19@tts.org for inclusion on our resources page.
In this dashboard, you will find links to TTS and other global and regional resources, as well as interactive maps, publications and webinars. We encourage you to explore this dashboard and share with your colleagues.
Website - www.tts.org/covid-19
Editors and contributors to Transplantation have shared their thoughts on how they are dealing with the current crisis. While we understand that the information of today may be quite different tomorrow in this fast-moving pandemic, this report will open our forum of an international exchange on COVID for the transplant community.
Website - www.tts.org/txjcovid19
Please send your own contributions and news to covid-19@tts.org for inclusion on our resources page.
TTS 2020 CALL FOR AWARDS ANNOUNCEMENT & NEW TTS STARZL INNOVATION AWARD
THE MEDAWAR PRIZE
Application Deadline - May 1, 2020
Recognized as the world's highest dedicated award for the most outstanding contributions in the field of transplantation.
TTS THOMAS STARZL INNOVATION AWARD
Application Deadline - May 1, 2020
The award recognizes individuals who, though advanced and original work, have contributed significantly to transplantation, thereby reflecting the spirit pioneered by Dr. Starzl.
TTS RECOGNITION AWARDS
Application Deadline - May 1, 2020
These awards recognize individuals who have made a major international impact in the field of transplantation.
WIT AWARDS
Application Deadline - May 1, 2020
The Woman Leader in Transplantation Award and Unsung Hero Award will be presented to women with extraordinary impact in the field.
KDIGO ANNOUNCES PUBLICATION OF THE TRANSPLANT CANDIDATE GUIDELINE
Brussels, Belgium — Kidney Disease: Improving Global Outcomes (KDIGO) announces the publication of its Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. The full guideline is published in the April issue of Transplantation Supplements, the official journal of The Transplantation Society. The full guideline and supplementary materials can also be found on the KDIGO website.
The guideline was developed using KDIGO’s traditional and rigorous process. A sixteen-member Work Group wrote the guideline and is solely responsible for its content. The Work Group was led by Steven J. Chadban of the Royal Prince Albert Hospital, University of Sydney, Australia and Gregory A. Knoll, Ottawa Hospital Research Institute, Ottawa, Canada. The work was assisted by an independent Evidence Review Team from Brown University School of Public Health, Providence, Rhode Island, USA
The guideline “recommends that all patients with chronic kidney disease who are expected to reach kidney failure be informed of, educated about, and considered for kidney transplantation regardless of socioeconomic status, sex, gender identity or race/ethnicity.”
The guideline also systematically examines current evidence concerning the risks of transplantation associated with specific conditions and provides recommendations as to how clinicians may wish to deal with specific risk factors. It covers both pediatric and adult transplantation as well as transplants from both living and deceased donors.
The recommendations for evaluation of individual aspects of a candidate’s profile enable clinicians to evaluate each risk factor and comorbidity separately. Estimation of risk is a key part of a transplant candidate’s evaluation. This guideline helps in the consideration of how to minimize risks and maximize the chances of a successful outcome.
In a preface to the guideline, KDIGO Co-Chairs Wolfgang Winkelmayer and Michel Jadoul remarked, “KDIGO seeks and considers all available evidence in producing guidelines which are of global relevance. However, the fact that the practice and outcomes of transplantation vary enormously across the globe requires the reader to consider their local practices and outcomes in interpreting and implementing the guideline.”
They added, “We thank the Work Group Co-Chairs and all members who volunteered countless hours of their time to develop this guideline. We also thank The Transplantation Society for their support in making this project possible.”
KDIGO is a Belgian foundation committed to developing and implementing nephrology guidelines that improve patient outcomes on a global basis.
For further information please contact KDIGO at KDIGOcommunications@kdigo.org.
IN CASE YOU MISSED LAST WEEK'S TTS-IPTA WEBINAR
TITLE: SOCIAL MEDIA USE IN PEDIATRIC TRANSPLANTATION
CLICK HERE TO VIEW THE RECORDING (YOU MUST BE LOGGED INTO THE WEBSITE)
TRANSPLANTATION - HIGHLIGHTED ARTICLE

Dr. Joel T. Adler, Editorial Fellow, Transplantation
Gender Disparities in Patients With Alcoholic Liver Disease Evaluated for Liver Transplantation
McElroy LM, Likhitsup A, Winder GS, et al.
Transplantation: February 2020 - Volume 104 - Issue 2 - p 293-298
There are a number of disparities in transplantation, leading to differential waitlisting and transplantation across differently advantaged groups. While the disadvantages incurred by the MELD score to females are well accepted, there is little is known about gender-based disparities in the evaluation and listing for liver transplantation for alcoholic liver disease.
In a single-institution study of patients with alcoholic liver disease evaluated for transplant, men were more likely to be listed compared to women (10% versus 19%; P < 0.05); this was despite an overall increase in the proportion of female candidates being evaluated with alcoholic liver disease. This gender disparity underlines the necessity for effective treatment to improve transplant eligibility and increase access to transplantation.
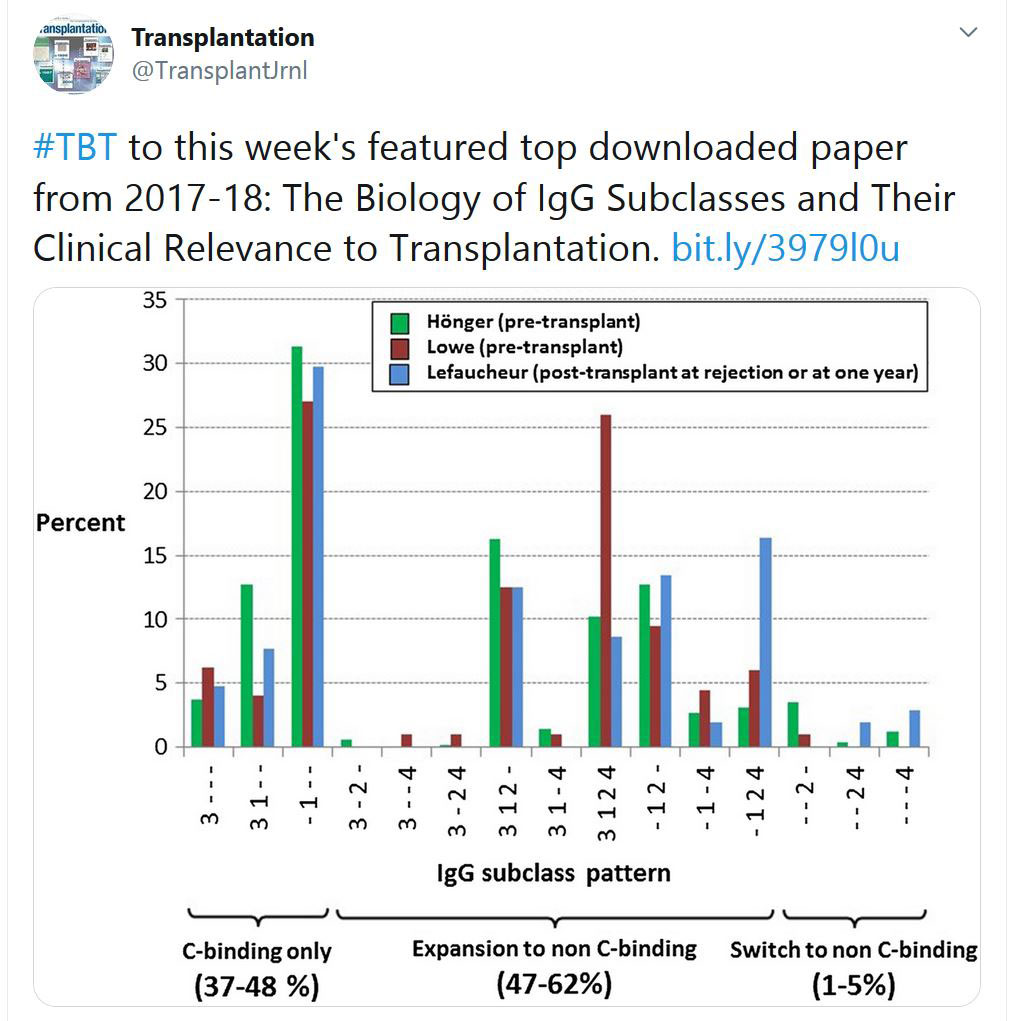
TRANSPLANTATION - WEEK'S MOST DOWNLOADED PAPER
IN THE NEWS
ADVANCED LIVER DISEASE PATIENTS AND TRANSPLANT RECIPIENTS NEED SPECIFIC CARE DURING COVID-19
April 2 - Patients with advanced liver disease and recipients of liver transplants represent vulnerable groups and are likely to be at an increased risk of infection and/or a severe course of COVID-19. To promote the best possible care in these challenging circumstances, this position paper provides recommendations for clinicians treating patients with chronic liver diseases.
FDA OKS 1ST STEM CELL HUMAN TRIAL FOR COVID-19
April 3 - A human clinical trial of an experimental stem cell therapy for coronavirus patients has been approved by the U.S. Food and Drug Administration. The stem cell treatment is derived from human placentas and is being developed by New Jersey biotech company Celularity. The early-stage trial will include up to 86 coronavirus patients with symptoms who will receive infusions of the stem cell therapy to assess the its safety and whether it prevents the patients from developing more severe illness.
FDA APPROVES 50CC TOTAL ARTIFICIAL HEART SYSTEM AS BRIDGE TO TRANSPLANTATION
April 1 - The Food and Drug Administration (FDA) approved the 50cc temporary Total Artificial Heart System (50cc TAH-t) developed and manufactured by SynCardia Systems, LLC as a bridge to heart transplant for patients who are at imminent risk for death from biventricular failure.
MIT DEVELOPS NEW MODEL FOR ORGANS-ON-A-CHIP AND DRUG RESEARCH

April 3 - Researchers at MIT have created a new model for organs-on-a-chip that could be a crucial step in developing treatments for inflammatory diseases and afflictions. Organs-on-a-chip essentially consist of millions of cells formed on a platform so they replicate the functions of different organs. These types of devices can be instrumental in analysing and potentially creating new treatments for complex diseases.
DIABETES MAY BE AN INDEPENDENT PREDICTOR OF SUDDEN CARDIAC DEATH AFTER LIVER TRANSPLANTATION

March 31 - The presence of diabetes before liver transplantation may be an independent predictor of sudden cardiac death after the procedure, according to study results intended to be presented at the American College of Cardiology’s 69th Annual Scientific Session.
UPCOMING MEETINGS AND ANNOUNCEMENTS
3rd IPITA/JDRF/HSCI Conference on Stem Cell Derived Beta Cells
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!