LAST CHANCE TO REGISTER!
REGISTRATION FOR IPITA, IXA, CTRMS AND TTS MEMBERS

Upcoming Webinars
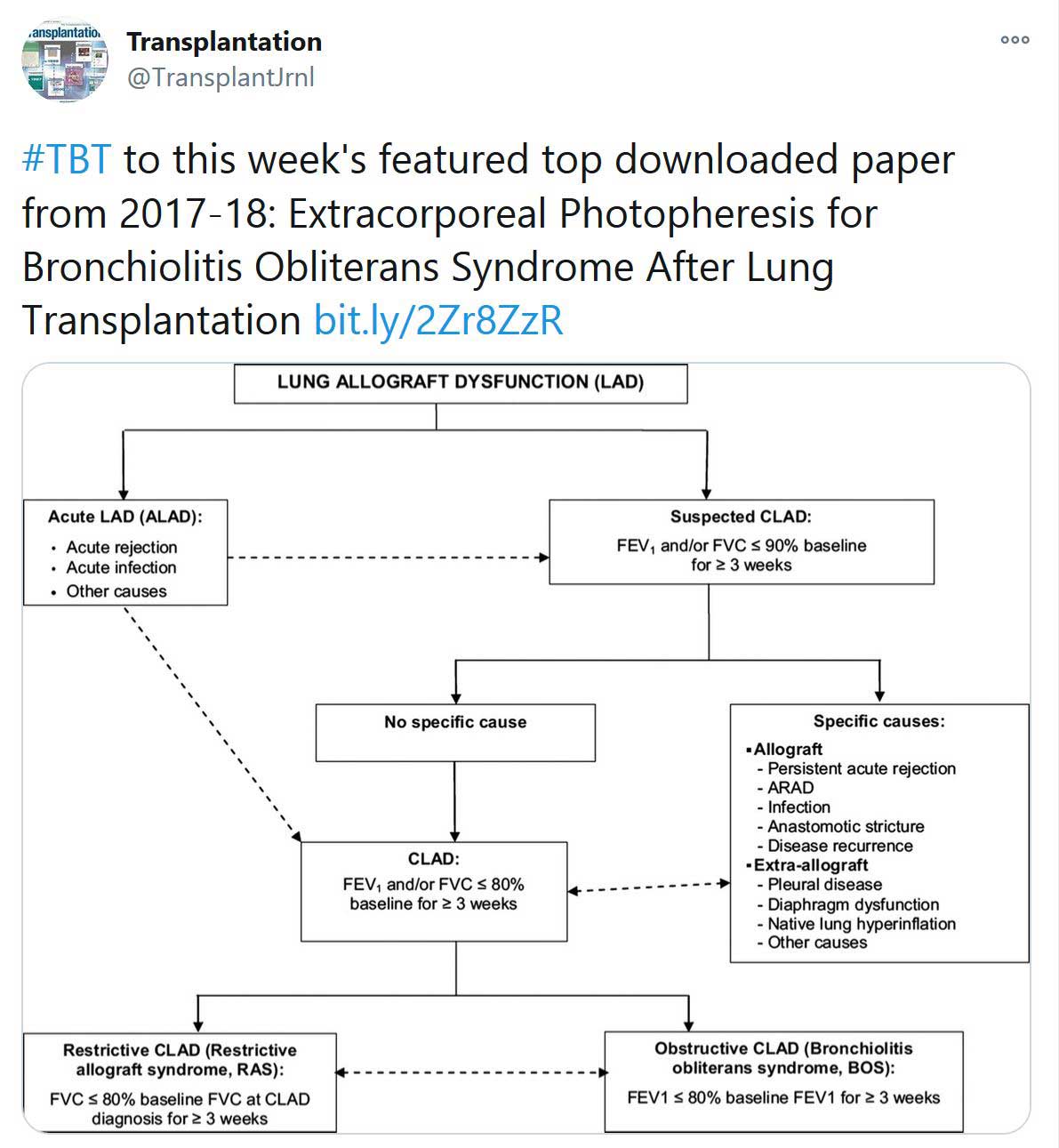
«HOT OFF THE PRESS»
RECENT PUBLICATIONS IDENTIFIED BY TTS EDUCATION COMMITTEE ON COVID-19
Selected Publications by TTS Education Committee. This week's selection made by Enver Akalin and Marlies Reinders
CORONAVIRUS (COVID-19) UPDATES
IN THE NEWS
UPCOMING MEETINGS AND ANNOUNCEMENTS
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!