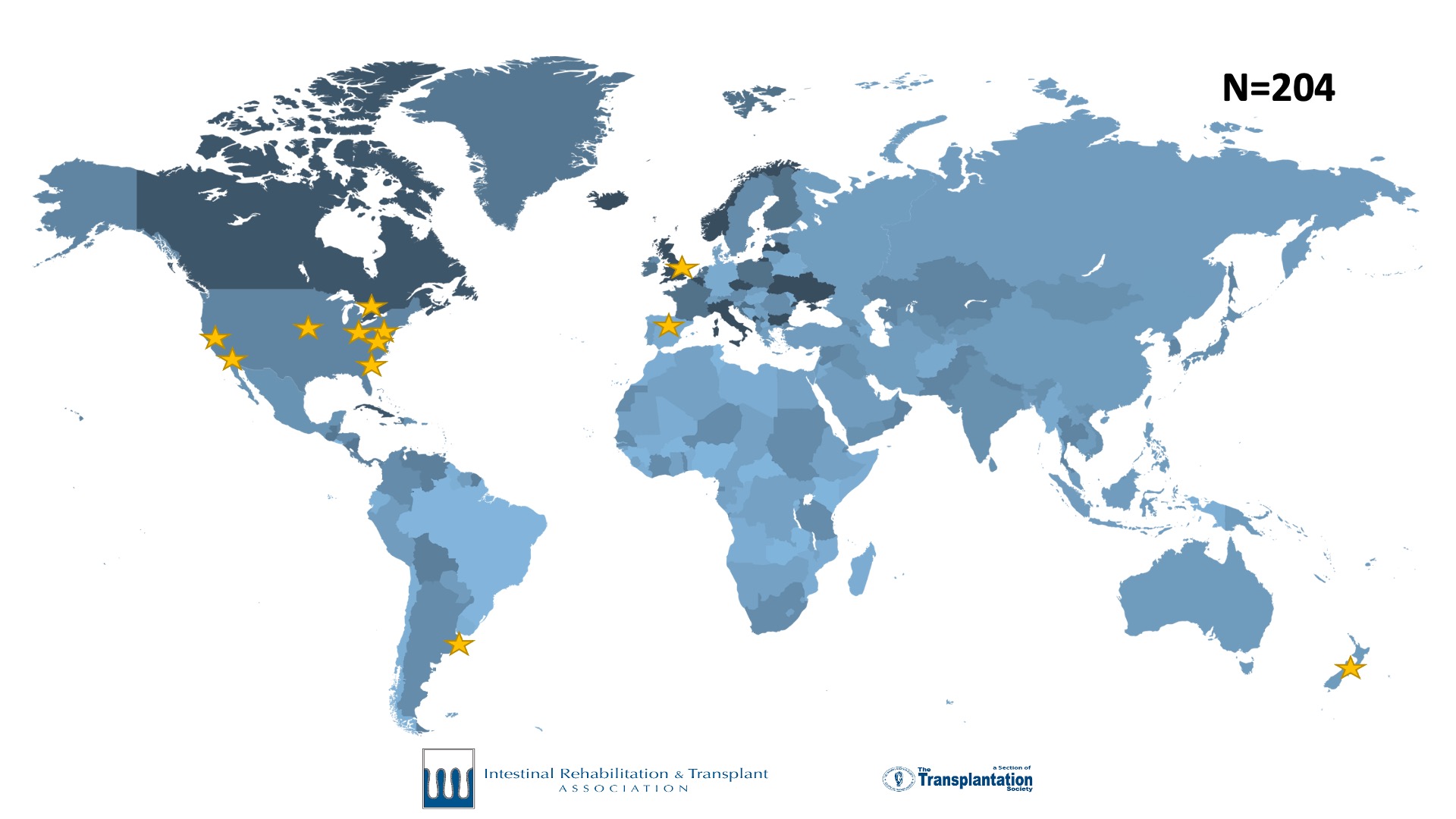
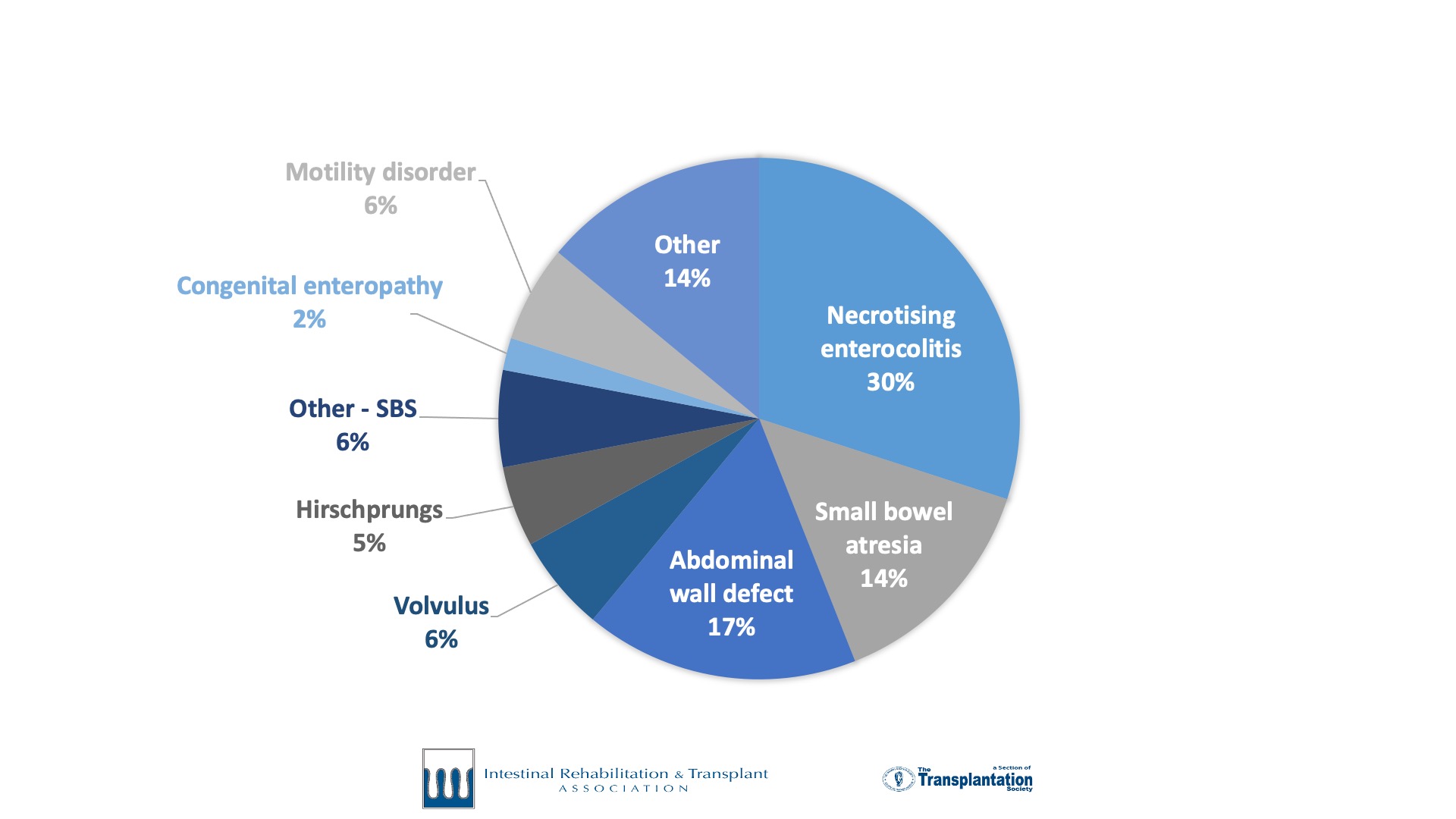
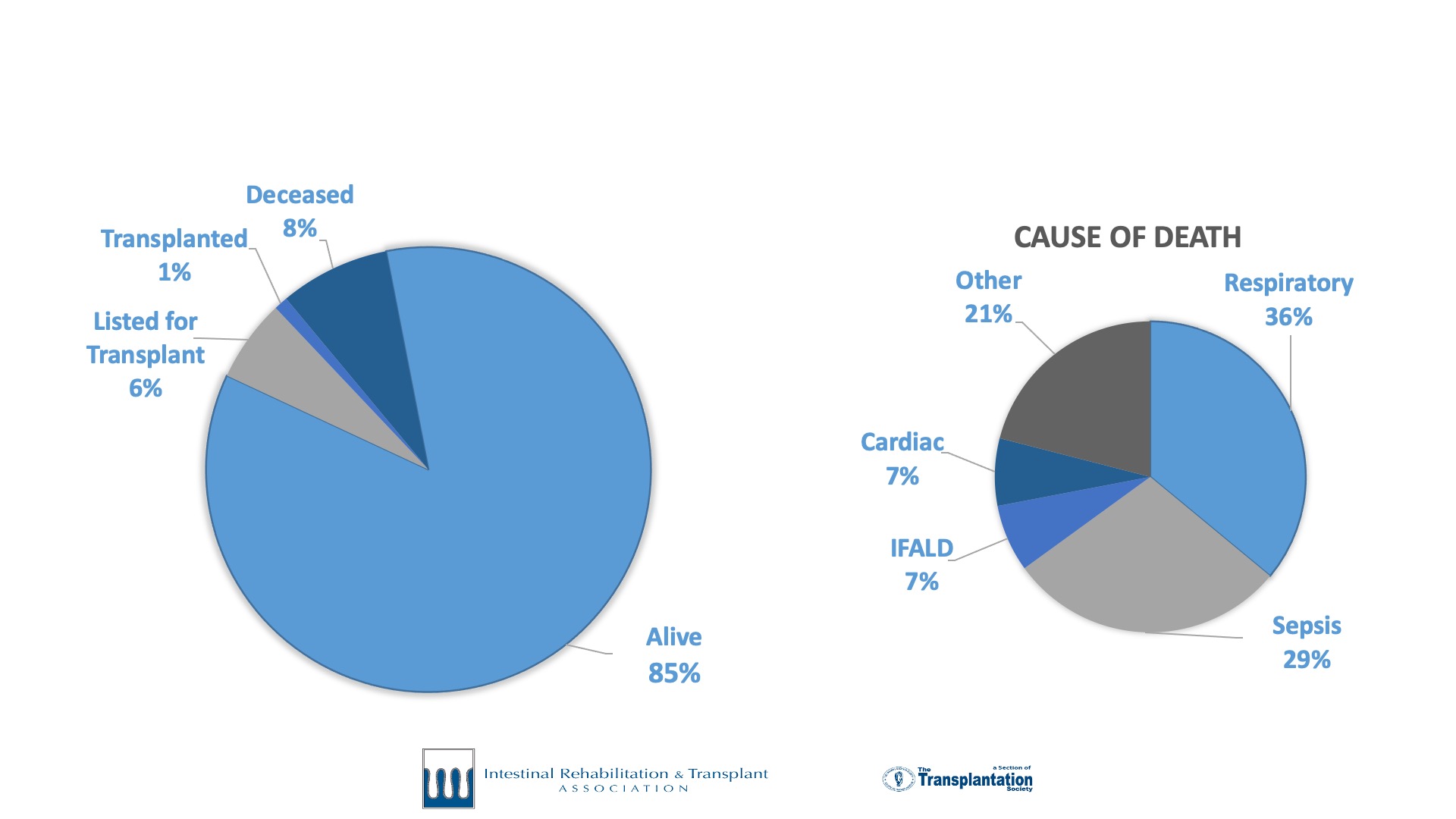
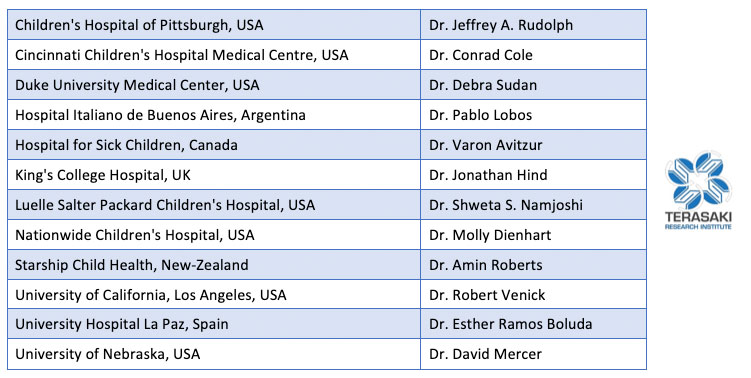
Intestinal failure is a rare disease. Accurate outcome data and research in intestinal failure has historically been limited by small patient volumes at individual centers. To meet the need for a global collaborative effort to track and report current trends in intestinal failure, the International Intestinal Rehabilitation and Transplant Association (IIRTA) established a dedicated registry for intestinal failure. Twelve centers participated in a successful pilot of the registry from 2018-2020. Following this phase, the IFR began collecting data in 2021.
The IFR is owned and managed by the IIRTA with support from The Transplantation Society (TTS). Scientific work is directed by a subcommittee of the Scientific Committee of the Association.
The IFR is endorsed by the North American Society for Pediatric Gastroenterology and Nutrition (NASPGHAN) and the American Society for Parenteral and Enteral Nutrition (ASPEN).
The IFR is supported by non-restricted and education grants by Takeda LTD and Stanford University & the Lucile Packard Children’s Hospital