2017 - IPITA
This page contains exclusive content for the member of the following sections: TTS, IPITA. Log in to view.
Pancreas Transplantation - Outcomes & Complications 1
5.2 - Simultaneous Pancreas and Kidney Transplantation - Outcomes and predictors of long-term patient and graft survivals
Presenter: Richard, Allen, Westmead, Australia
Authors: Richard Allen, Henry Pleass, Germaine Wong, Paul Robertson, Wayne Hawthorne, Kathy Kable, Brendan Ryan, Brian Nankivell, Philip O'Connell, Jeremy Chapman
Simultaneous Pancreas and Kidney Transplantation - Outcomes and predictors of long-term patient and graft survivals
R. Allen1,2, H. Pleass1,2, G. Wong1,2, P. Robertson1, W. Hawthorne1,2, K. Kable1, B. Ryan1, B. Nankivell1,2, P. O'Connell1,2, J. Chapman1,2.
1National Pancreas Transplant Unit, Westmead Hospital, Sydney, Australia, ; 2Sydney Medical School, University of Sydney, Sydney, Australia,
Background: Simultaneous pancreas kidney (SPK) transplantation is the treatment of choice for young patients with Type 1 Diabetes Mellitus (DM1) and end-stage kidney disease (ESKD). While the short to medium term outcomes are well-described, long-term graft and patient survival and the predictors of graft loss are under-studied.
Methods: Using data from the Australian National Kidney Pancreas Transplant Unit registry, overall graft and patient survival rates stratified by year of transplantation were estimated using Kaplan-Meier methods. Multivariate Cox proportional hazard regression modelling was conducted to determine the predictors of patient and graft survival.
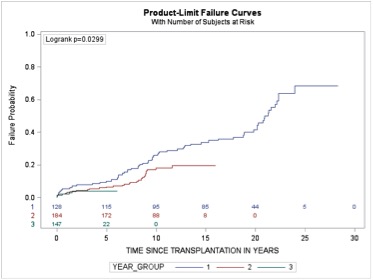
Results: A total of 454 simultaneous pancreas kidney transplant (SPK) recipients were followed over 3274 person-years with the longest to 28.3 years. The average (standard deviation (SD)) recipient age was 38.9 (7.0) years and 257 (59%) were men. The mean (SD) donor age was 26.8 (9.7) years, with average donor BMI of 23.7 (4.1) kg/m². All patients received systemic venous drained whole pancreas grafts with pancreas and kidney mean total ischemia times and vascular anastomosis times of 10.9 and 11.0 hours, and 30.0 and 32.7 minutes, respectively. All recipients received subcutaneous heparin as inpatients and daily aspirin after discharge. The majority (73.8%) involved exocrine drainage to distal small bowel. A total of 88 (19.4%) and 57 (12.6%) recipients lost their pancreas and kidney allografts over the median follow-up time of 6.44 years, with more than 50% of the pancreas graft losses occurring within the first three months of transplantation. The primary causes of early pancreas allograft loss were thrombosis (31, 70.4%) and rejection (9,20.4%). The overall 5, 10, 15 and 20-year pancreas and kidney allograft survivals were 83%, 77%, 67% and 50%; 94.5%, 85%, 71% and 52%, respectively. The overall patient survival at 5, 10, 15 and 20 years after transplantation was 90%, 78%, 71% and 56%, respectively. Of the 99 recipients who died during follow-up, almost half died from vascular disease (n=48, 48%), followed by cancer (25, 25.2%) and infection (13, 13.1%). Recent era of transplantation (per year increase) [adjusted HR (95%): 0.96 (0.92 – 0.99)] and male donors [(adjusted HR (95%): 0.56 (0.31 – 0.99)] were predictors of better pancreas and kidney graft survival, respectively. Younger age at transplantation (per year decrease) [(adjusted HR (95%): 0.95 (0.92 – 0.98), transplantation in recent era (per year increase) [0.96 (0.93 – 0.99) (figure 1) and the use of left-sided donor kidneys [0.53 (0.29 – 0.95)] were associated with a lower risk of death.
Conclusions: SPK has proved to be an enduring procedure, particularly for younger patients at time of transplantation. Age at transplantation is a critical factor and attempts to increase recipient age criteria cannot be justified. Improved outcomes over time can be attributed to improved immunosuppression and its management. Cardiovascular disease and malignancy, the principle causes of long-term death, are modifiable risk factors likely to affect all SPK recipients. These two factors warrant aggressive screening, intervention and life long multi-disciplinary surveillance from time of recipient wait-listing for SPK transplantation.
Figure 1. Probability of patient death by era - Era1 = 1987-2000, Era 2 = 2001-2010, Era 3 = 2011-2016
Important Disclaimer
By viewing the material on this site you understand and accept that:
- The opinions and statements expressed on this site reflect the views of the author or authors and do not necessarily reflect those of The Transplantation Society and/or its Sections.
- The hosting of material on The Transplantation Society site does not signify endorsement of this material by The Transplantation Society and/or its Sections.
- The material is solely for educational purposes for qualified health care professionals.
- The Transplantation Society and/or its Sections are not liable for any decision made or action taken based on the information contained in the material on this site.
- The information cannot be used as a substitute for professional care.
- The information does not represent a standard of care.
- No physician-patient relationship is being established.
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!
