TID 2022 Virtual - 14th International Transplant Disease Conference
C-Path - Transplant Therapeutics Consortium (TTC) Press Release
C-Path’s Transplant Therapeutics Consortium Receives
EMA Draft Qualification Opinion for iBox Scoring System
About TTS and the Transplantation Therapeutics Consortium (TTC)
Since 2017 TTS has been participating in the Transplantation Therapeutics Consortium (TTC), a program of the Critical Path Institute (https://c-path.org/programs/ttc/).
TTC is a public-private collaboration between the transplant community, including industry, academia, professional societies, and regulatory agencies. TTC’s mission is to accelerate the pace of medical product development for transplant recipients with a focus on obtaining regulatory agency endorsement of new medical products. TTS members, Roslyn Mannon and Randy Morris have been representing TTS in this forum. TTC’s initial work has been on endorsing the iBox Scoring System (Composite Biomarker Panel) for acceptance by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as a surrogate endpoint to accelerate the pace of drug development in the field of kidney transplantation.
Transplantation Updates

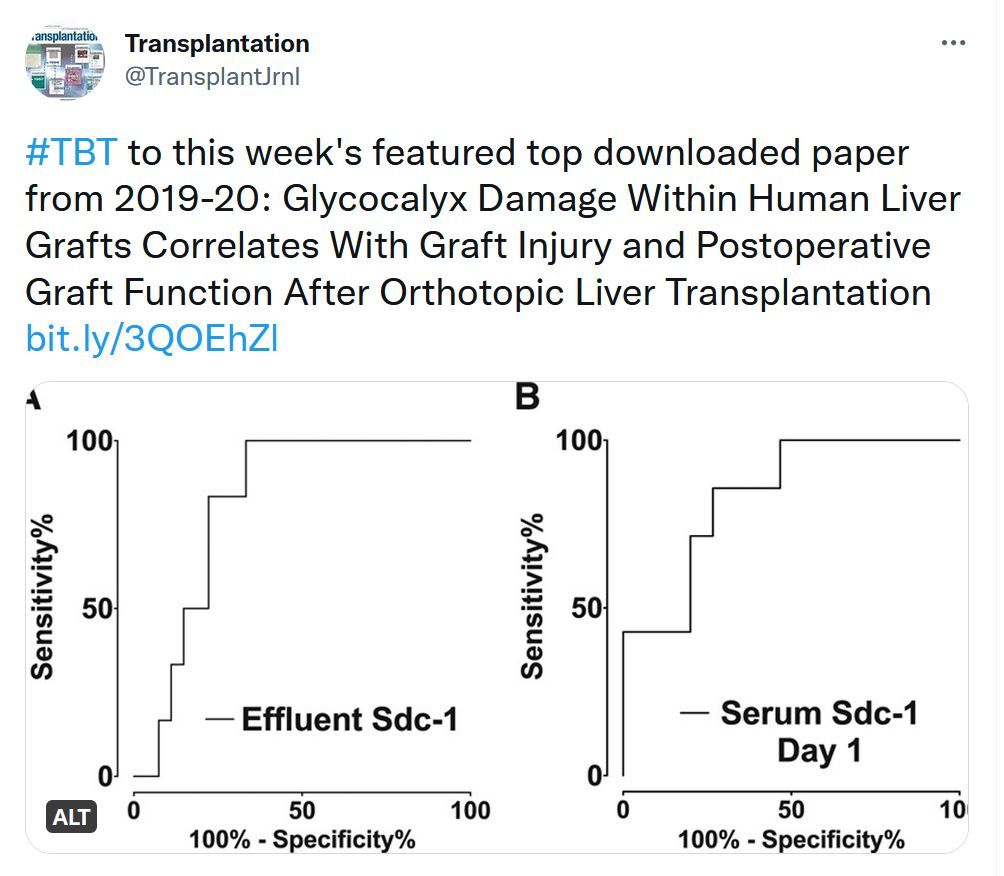
Transplantation - Week's Most Downloaded Paper
Glycocalyx Damage Within Human Liver Grafts Correlates With Graft Injury and Postoperative Graft Function After Orthotopic Liver Transplantation
Destruction of the endothelial glycocalyx has been observed within lung and kidney grafts during ischemic organ preservation. We aimed to quantify glycocalyx damage within human liver grafts after organ preservation and correlate the results with graft injury and postoperative graft function in patients undergoing orthotopic liver transplantation (OLT).Transplantation Direct - Highlighted Tweet
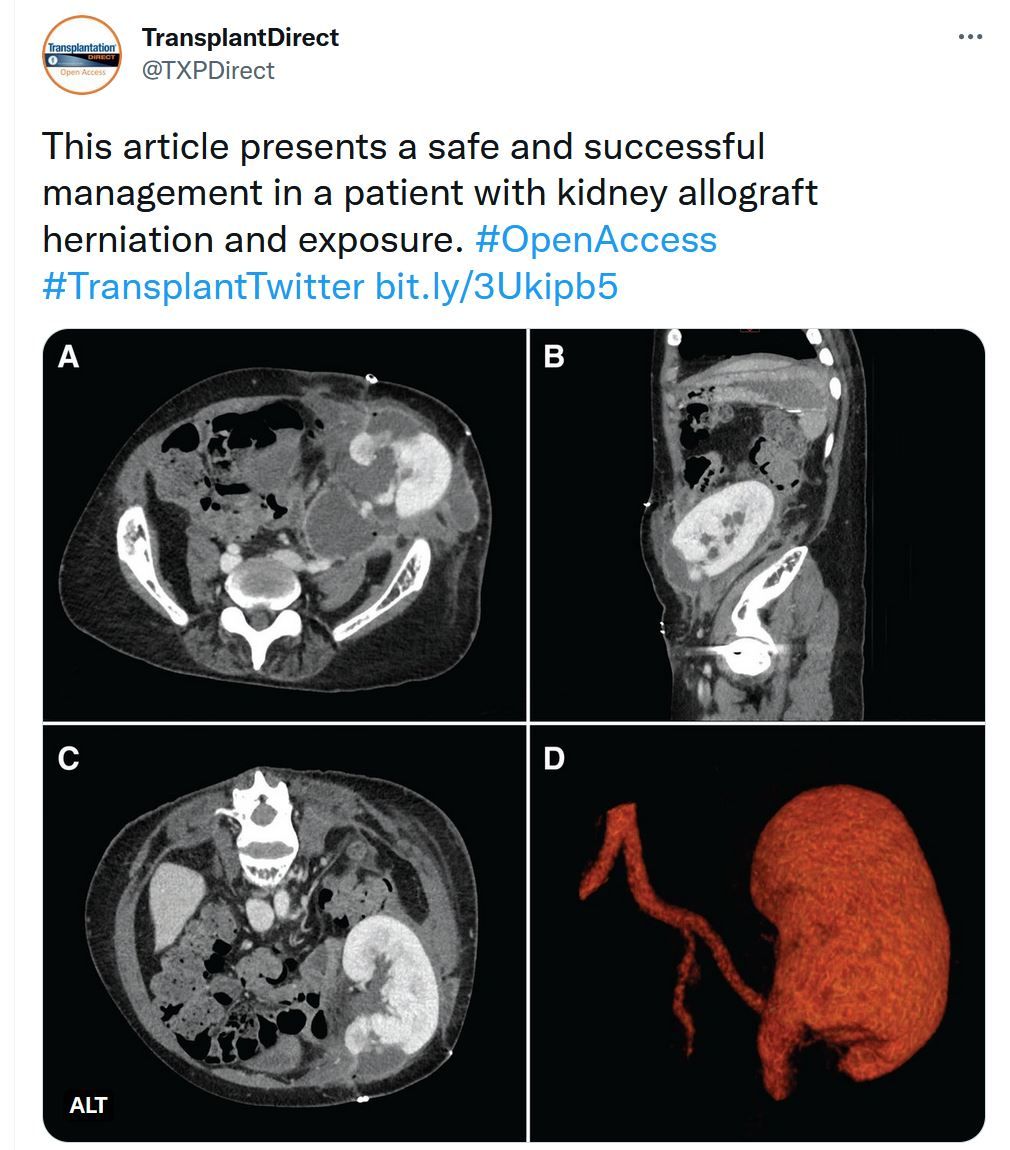
Challenges in the Management of Kidney Allograft Herniation With a Single-stage Pedicled Anterolateral Thigh Flap
Wound complications are the most common surgical complication after kidney allograft transplantation. Total wound rupture exposing the entire kidney is a rare and not well-described event. We present a successful treatment of this complication in a patient admitted to our unit. A single-stage procedure was performed combining debridement and reconstruction with a pedicled anterolateral thigh flap and an iliotibial band transferring. A short literature review is performed comparing the different treatment strategies and results.TTS-ILTS Paired Transplant Centers Program - Apply Today!
TTS-RMEI Partnership CME Activity
CMV is one of the most common opportunistic infections affecting solid organ transplant recipients (SOTRs), conveying higher risks of complications, graft loss, morbidity, and mortality. Treatment options for CMV are limited, have similar mechanisms of action, suffer from cross-resistance, and have serious toxicities. Until recently, no treatment option was available in the resistant/refractory setting. This workshop, developed in collaboration with The Transplantation Society (TTS) and Dr. Camille Kotton, MD, FIDSA, FAST, will take you through a CMV primer and then move on to discuss cases submitted by your peers from transplant centers across the United States.
Upon completion of this activity, participants should be better able to:- Employ evidence-based antiviral treatment strategies for cytomegalovirus (CMV) in solid organ transplant (SOT) patients
- Utilize antiviral resistance testing to identify SOT patients with treatment refractory or drug resistant CMV
- Implement new treatment strategies in the management of resistant or refractory CMV in SOT patients
CME:
Physicians - maximum of 0.75 AMA PRA Category 1 Credit(s)™
Nurses - 0.75 ANCC Contact Hour(s) (0 contact hours are in the area of pharmacology)
Pharmacists - 0.75 Knowledge-based ACPE (0.075 CEUs)- CME / CE Released: 9/15/2022 Valid for credit through: 9/14/2023
TTS Needs Assessment Survey - last chance to submit!
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!