Get Ready for the Virtual TTS 2024 Program Starting Monday!

We are excited to announce that the virtual portion of TTS 2024 kicks off this Monday! We can’t wait to welcome everyone to what promises to be an engaging and insightful event. We’ve updated the program to ensure the best experience for our virtual attendees, and we encourage you to explore the exciting sessions and speakers lined up.
5th Summit on Stem Cell Derived Islets - Online Registration Closes October 22nd!
IPTA Newsletter - October 2024
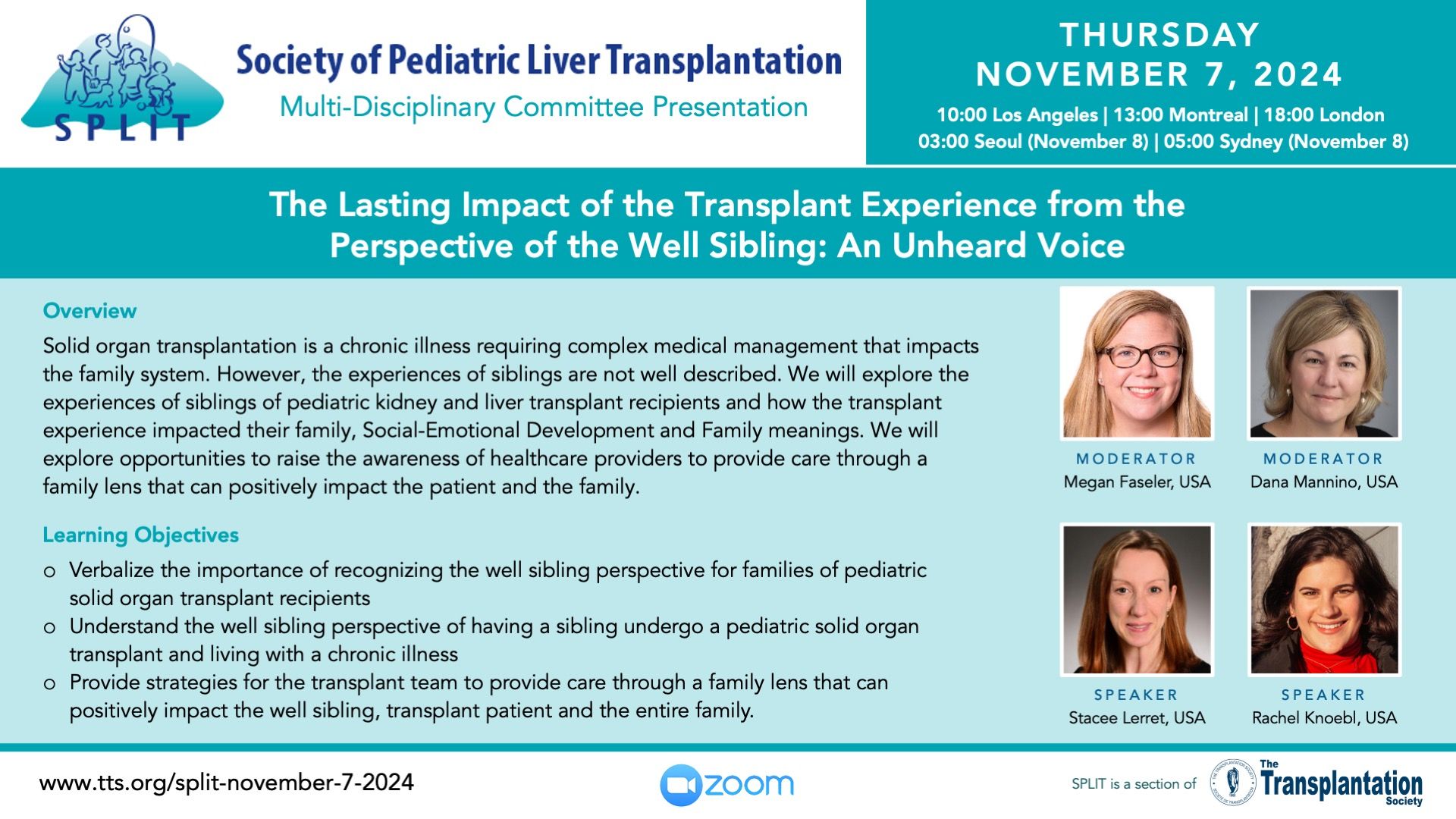
Upcoming Webinar Presentations
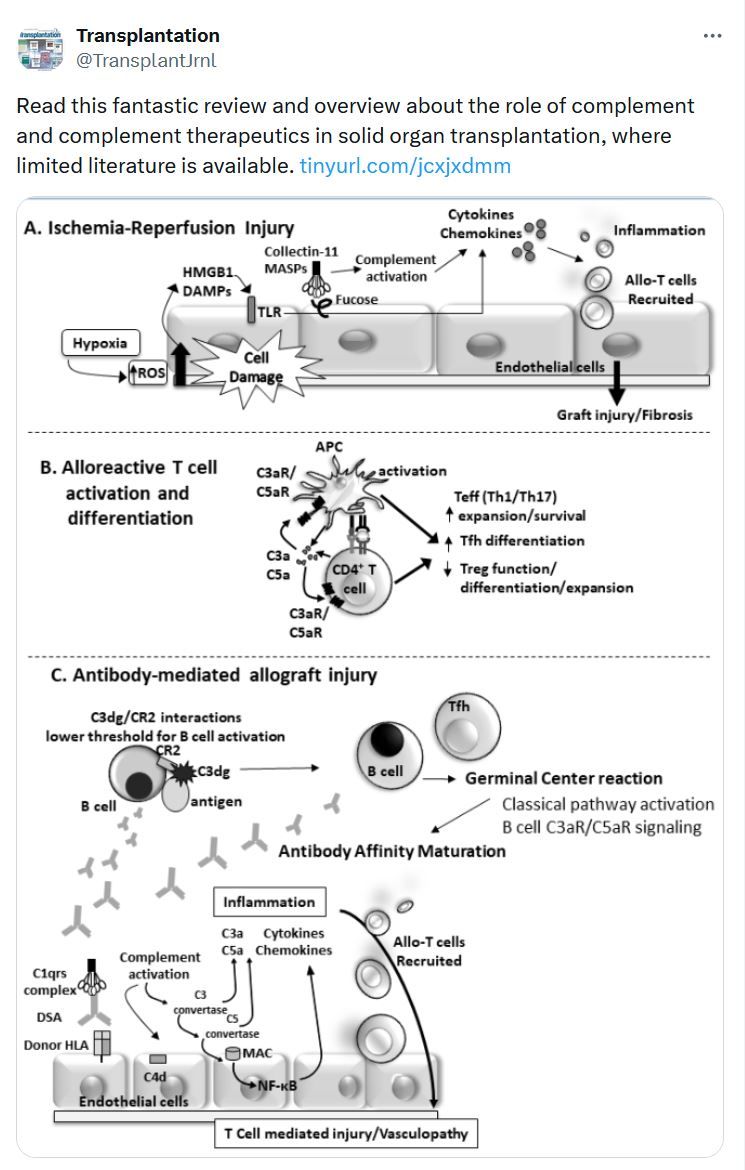
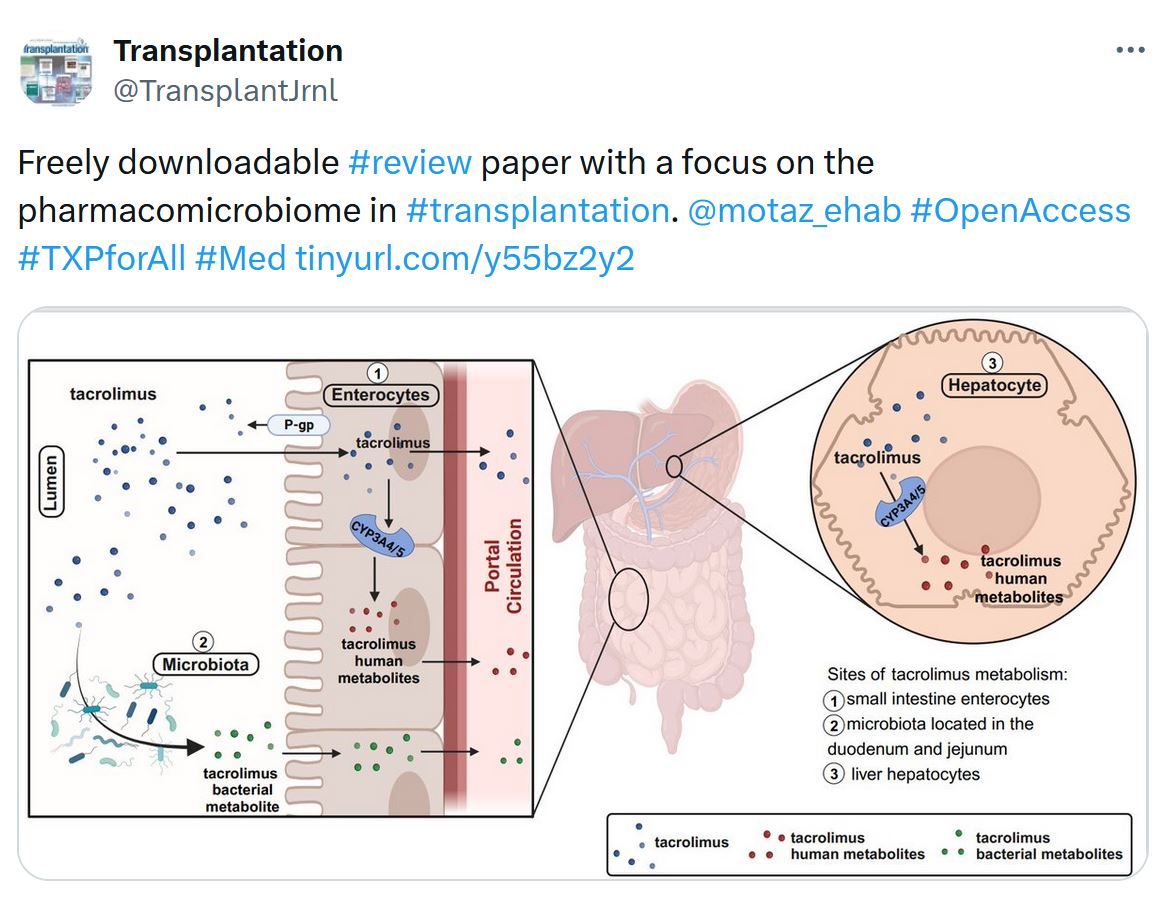
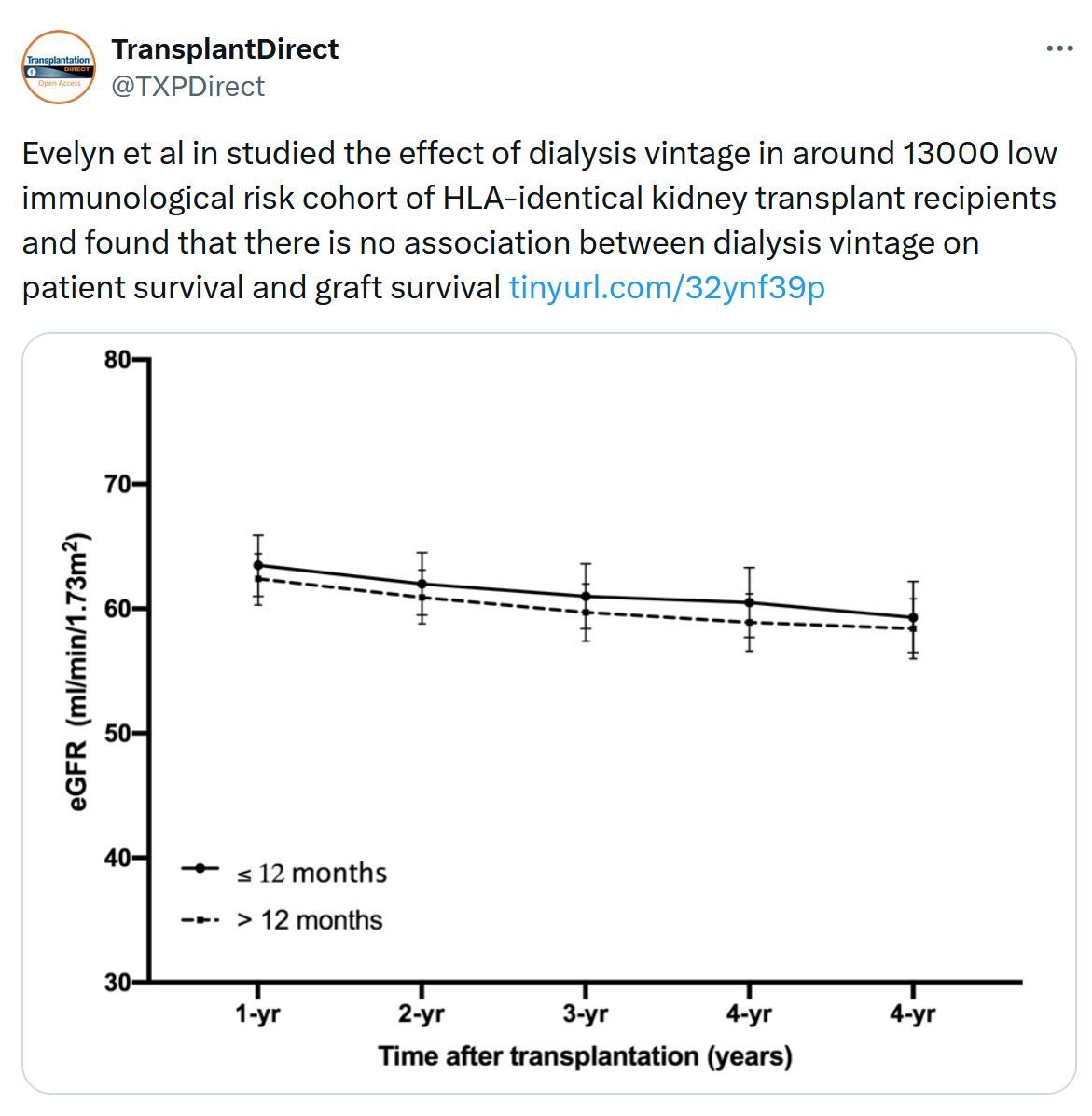
In Case You Missed It...
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!