Special IXA Feature

See Our International Xenotransplantation Association Sessions from TTS 2024
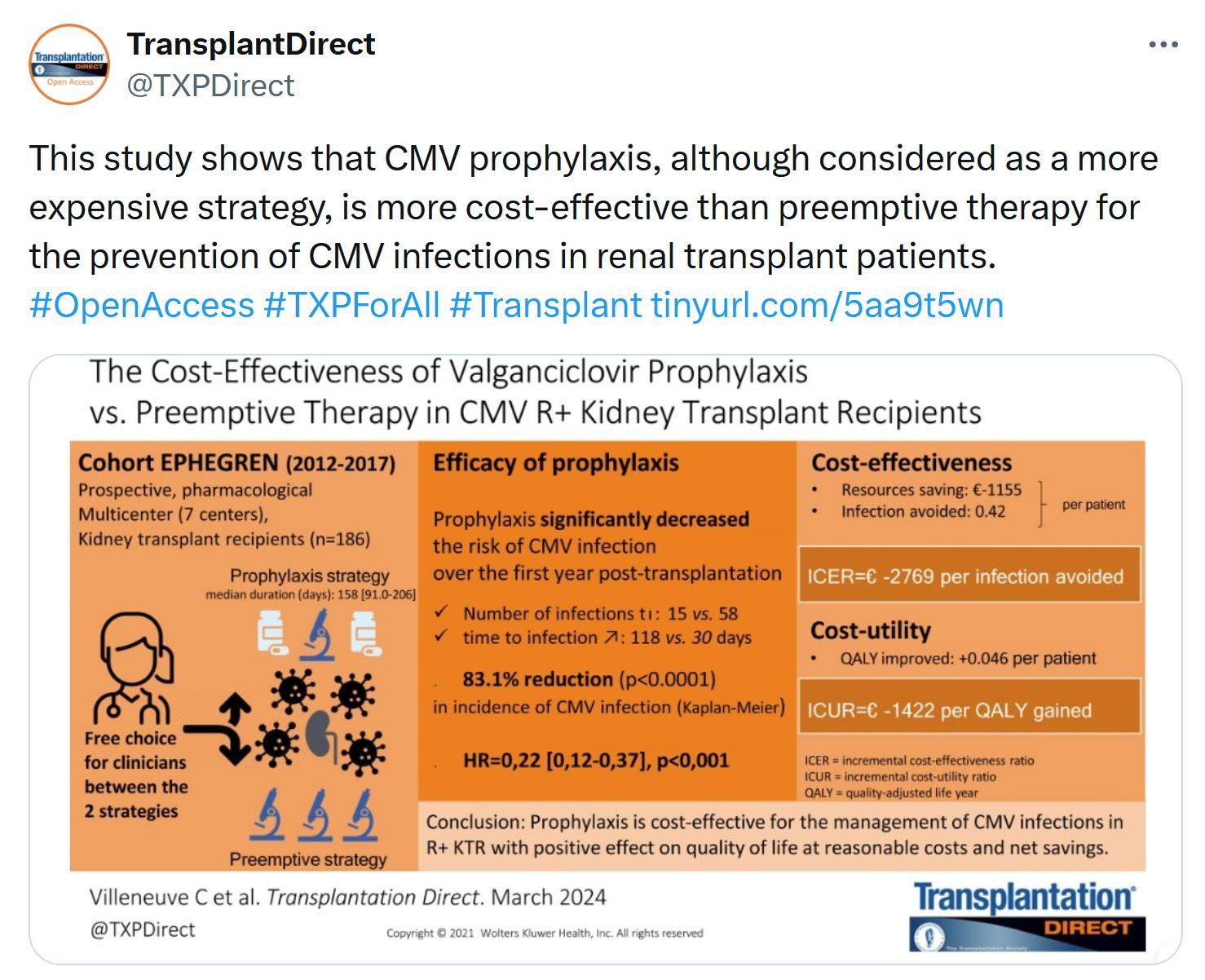
Just Released - Transplantation Direct - November Issue
Letter to the Editor
Kidney Transplantation
- Measurement Matters: A Metrological Approach to Renal Preimplantation Biopsy Evaluation to Address Uncertainty in Organ Selection
- Access to Waitlisting and Posttransplant Outcomes in Patients With Failed Kidney Allografts Secondary to Recurrent Glomerulonephritis
- Multifaceted Control Interventions for Healthcare-associated Infections in a Kidney Transplant Intensive Care Unit: Clinical Outcome Improvement and Bundle Adherence
- Spatial Transcriptomic Signatures of Early Acute T Cell–mediated Rejection in Kidney Transplants
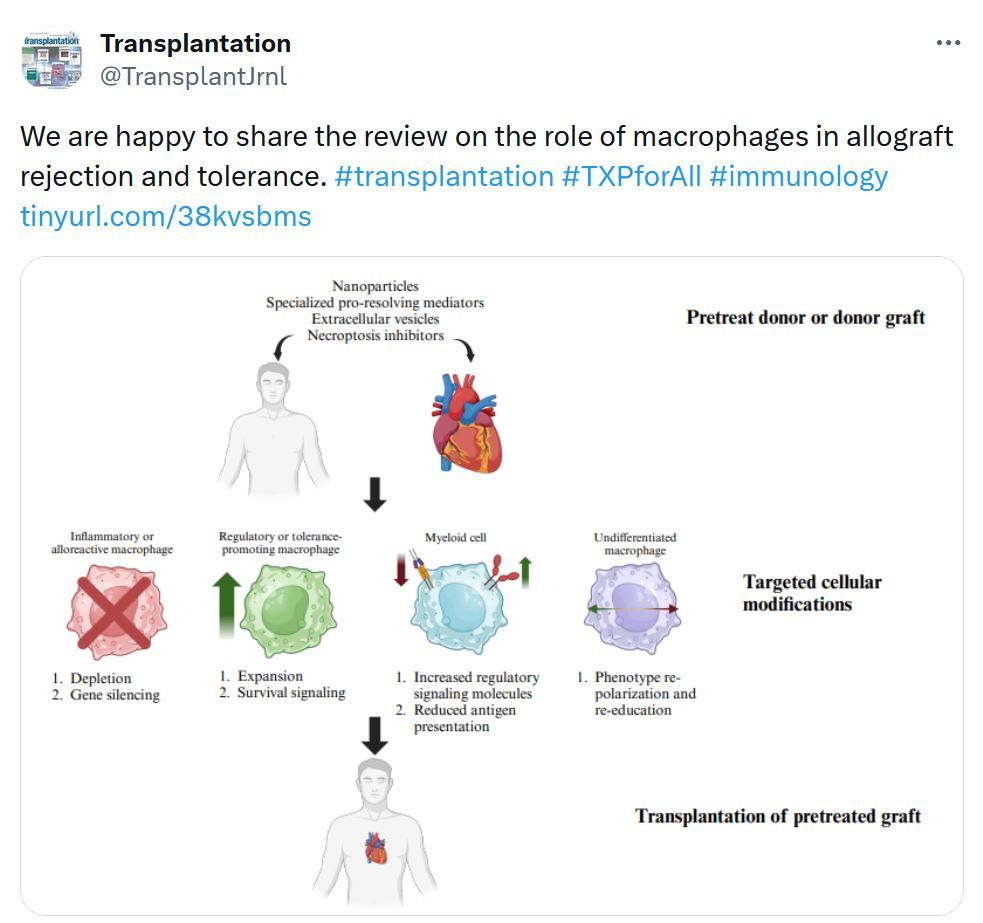
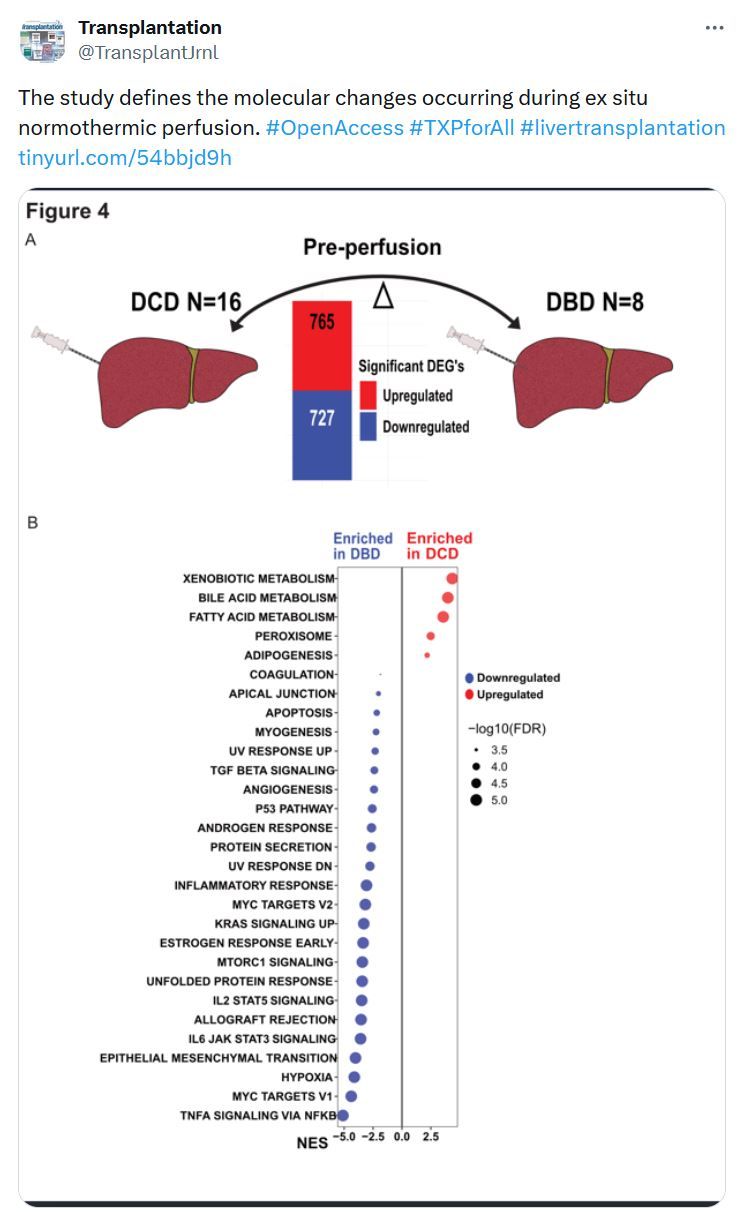
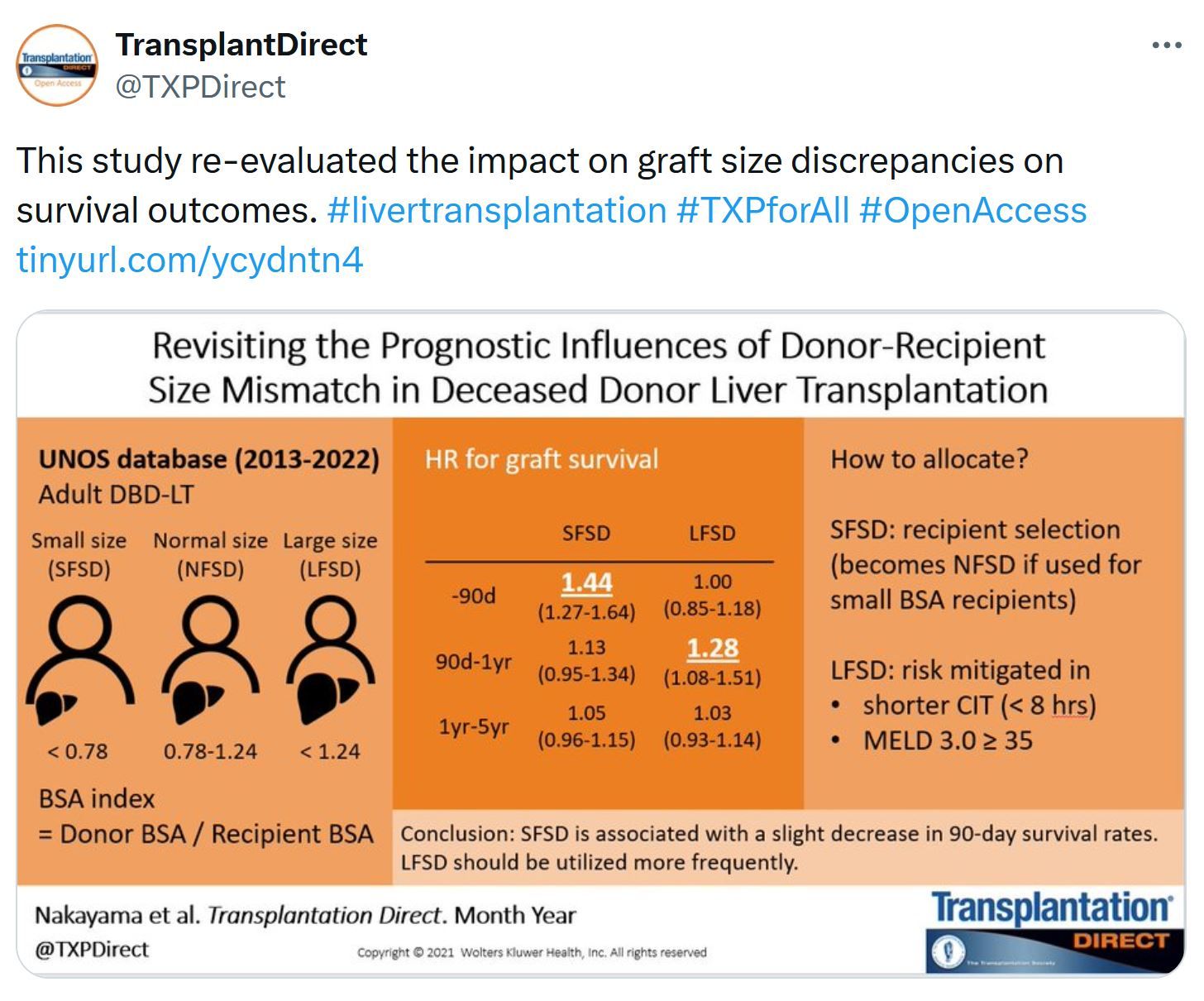
Liver Transplantation
Pancreas and Islet Transplantation
- Simultaneous Pancreas-Kidney Transplant Outcomes Stratified by Autoantibodies Status and Pretransplant Fasting C-peptide
- Risk Factors for Early Post-transplant Weight Changes Among Simultaneous Pancreas-kidney Recipients and Impact on Outcomes
Organ Donation and Procurement
- Nova Scotia’s Deemed Consent for Deceased Organ Donation: Family Member Perspectives and Experiences in the ICU Setting
- Early Complications in Kidney Donors and Course of Health-related Quality of Life 12 mo After Donation: An Analysis of the Swiss Organ Living-Donor Health Registry
Laboratory Method

Open to all IPTA and TTS Members
- The International Pediatric Transplant Association (IPTA) Virtual Education Symposium is open to IPTA and TTS members.
- This interactive symposium is over two half days on Thursday and Friday 14 and 15 November 2024, commencing with two separate streams for medics and for allied health and nursing professionals.
- This is an educational symposium which aims to cover both basic and advanced subjects for all trainee and staff physicians and surgeons and as well as members of the allied health and nursing professionals, including psychosocial teams.
- Basic and advanced immunology and immunosuppression
- Post-transplantation rejection and infection
- Post-transplant lymphoproliferative disorder
- Latest clinical information on nutrition, adherence, quality of life and sexual health
- Psychosocial aspects of transplantation
- Ethical and practical aspects of transplantation in low resource settings
- When to accept deceased donor organs for transplantation
- Academic guidance on writing grants, abstracts and manuscripts

In support of improving patient care, this activity has been planned and implemented by Amedco LLC and International Pediatric Transplantation Association (IPTA). Amedco LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Amedco Joint Accreditation #4008163.
Professions in scope for this activity are listed below.
Physicians
Amedco LLC designates this live activity for a maximum of 8.00 AMA PRA Category 1 CreditsTM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Nurses
Amedco LLC designates this activity for a maximum of 8.00 ANCC contact hours.
American Board for Transplant Coordinators
The American Board for Transplant Certification (ABTC) has approved this educational offering for up to 8.00 Category 1 Continuing Education Points for Transplant Certification (CEPTCs).
ABSTRACT SUBMISSION DEADLINE: OCTOBER 31
Successful applicants will be notified by Nov 8thPlease submit abstracts here:
SUBMIT YOUR ABSTRACT
Guidance on abstract submission:
- Abstracts will not be accepted unless conform to these guidelines.
- Please submit abstracts, written in English with Arial font 10 in Word document with abstract title limited to 30 words (without abbreviations in title) and abstract length up to 500 words (including any pictures, charts or tables noting only one jpg picture, chart or table allowed) with up to five total authors.
- Please state the presenting author and if this author is trainee physician / trainee surgeon / medical student / allied health professional / nursing professional / student nurse / student allied health professionals.
- Please state category of abstract: kidney / liver / heart / lung / liver / stem cell / bone marrow / multi-organ / ethics / psychosocial.
- Please structure your abstract as Aims / Methods / Results / Conclusions.
In case you missed it ... TTS2024 Recordings

ISN-TTS Sister Transplant Program Presentation
Upcoming Webinar Presentations
In Case You Missed It...
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!