TTS Participation at CAST 2025: Report from the Allied Health Sessions
Women in Transplantation Research Grants - Applications Now Open
On behalf of the entire Women in Transplantation Executive, I’m very excited to announce WIT funding opportunities for 2026. This will be the sixth year that we are offering research fellowships in sex and gender in solid organ transplantation.
These fellowships will support the next generation of scientists, furthering our understanding of the importance of sex and gender in transplantation. This is a growing field of interest and the more we learn the closer we are to applying these findings to clinical care to benefit patients and donors.
We are delighted to announce that we will continue to offer a grant to faculty working in low or middle income countries. This grant will allow transplant professionals working in low and middle income countries to work with an experienced mentor from among the WIT membership to address an important question within their own unique context. We hope that this award will spur exciting international collaborations and build more research capacity in lower resource settings.
For the second year, we are delighted to announce that we will run the WIT Research Grant for Projects in Gender and Sex and SOT amongst Indigenous Populations.
quests for Applications for all grants are now open and are being considered. Please note that we will accept only one mentee application per mentor, and only one application from any single administering institution (defined either by the hospital network or the university or academic institution. This will apply across all grants
If you have any further questions and for information on how to apply, please contact Katie Tait (WIT Manager) at katie.tait@tts.org to request more information.
We welcome all applications and look forward to an exciting competition!
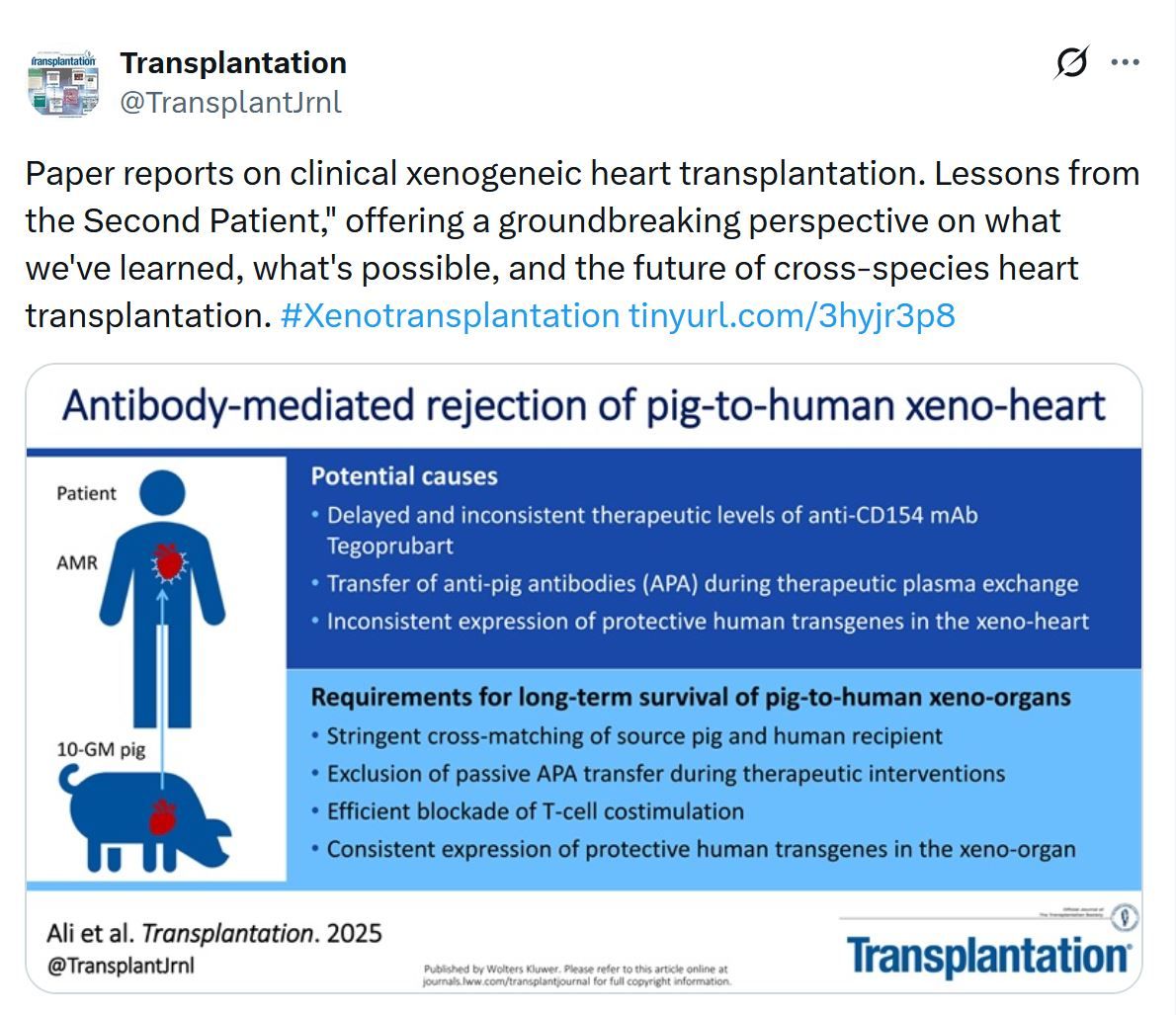
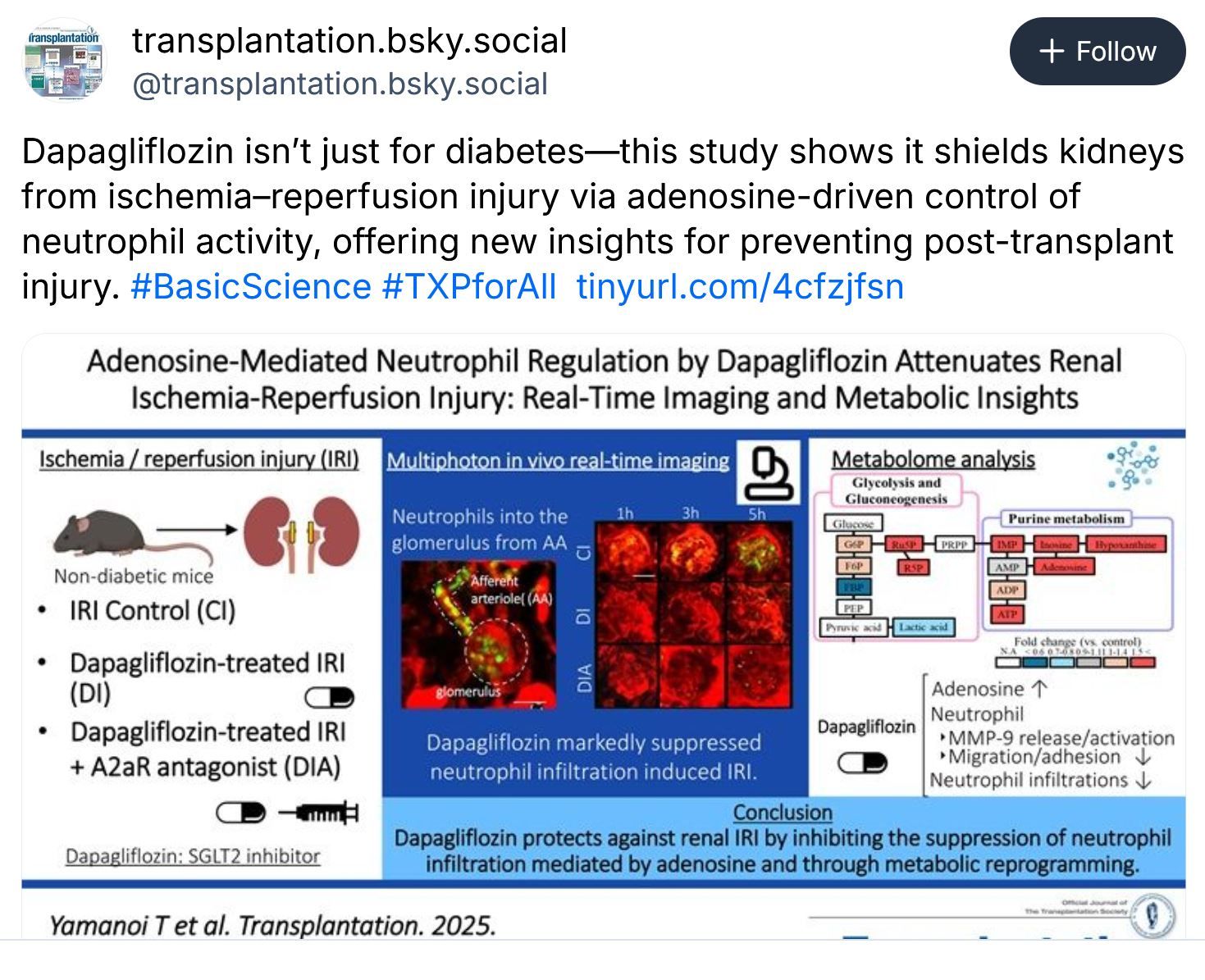
Just Released - Transplantation Journal - December 2025
In this December issue of Transplantation, we spotlight new data on transplant tourism—still accounting for an estimated 10% of global transplants—with Kelly Terlizzi and colleagues detailing U.S. patient travel patterns and motivations. Choisy et al. propose a timely combinatorial donor–recipient risk model that may reshape organ allocation beyond organ quality alone. Ethical questions in the rapidly expanding field of uterus transplantation are thoughtfully examined by Anji Wall and the International Uterus Transplant Society’s ethics committee. Sha and co-workers provide reassuring evidence from the US/Canadian Transplant Pregnancy Registry that assisted reproductive technologies are safe and effective for women after kidney transplantation. These selections reflect the breadth and depth of expert insights in the December issue—offering valuable perspectives for the entire transplant community.
Enjoy this issue of the journal. Happy holidays and all the best for 2026!
Stefan G. Tullius, MD, PhD
Editor-in-Chief, Transplantation journals
CLICK HERE TO ACCESS THIS ISSUE
TTS MEMBERS - CLICK HERE TO SIGN-IN FOR OPEN ACCESS THROUGH TTS.ORG
Special ISUTx Feature


Such a registry would enable systematic tracking of pregnancy course, neonatal complications, and child development into adulthood, as well as facilitate the early identification of late-emerging conditions. Beyond the scientific value, a registry is ethically necessary.
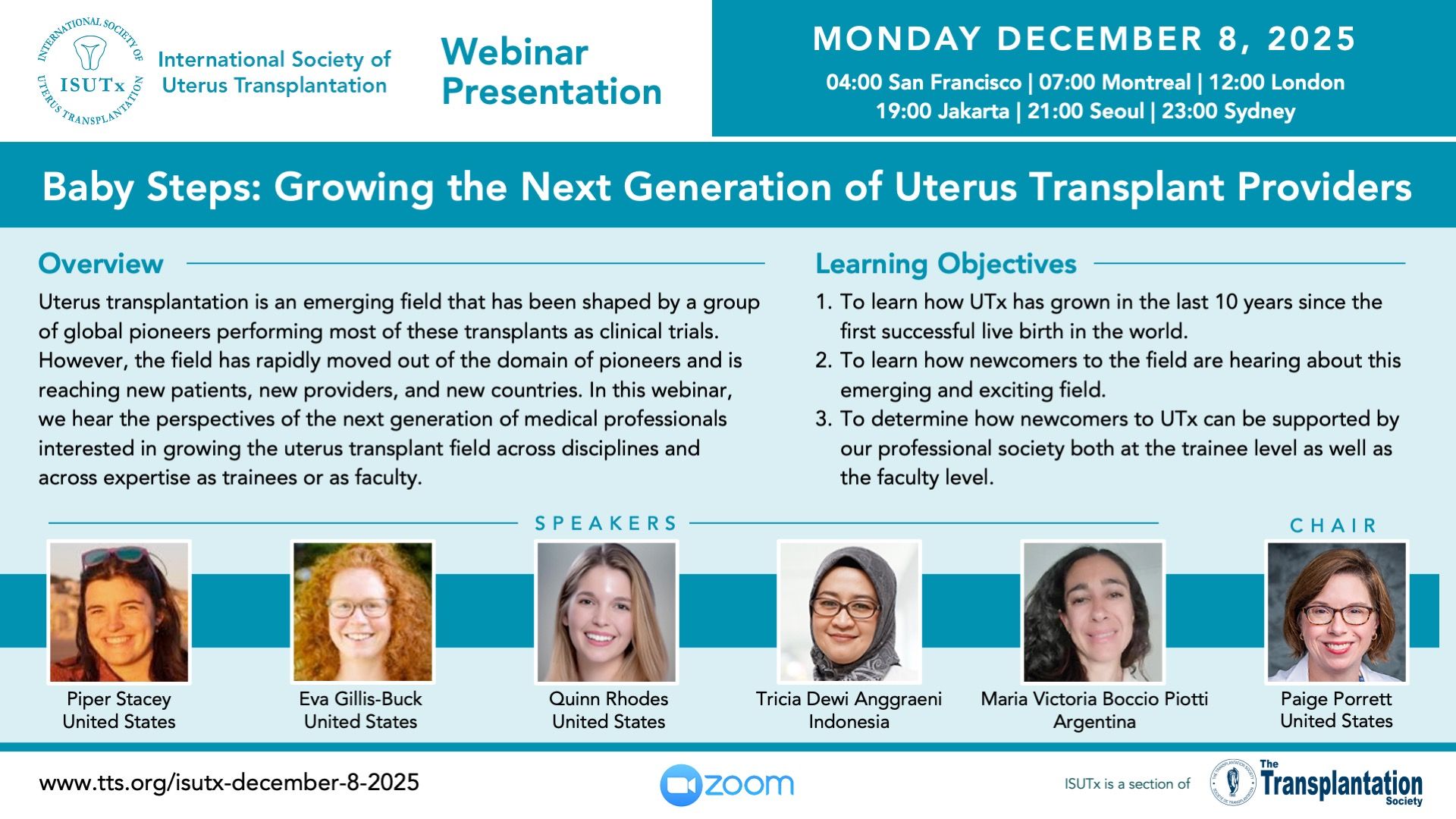
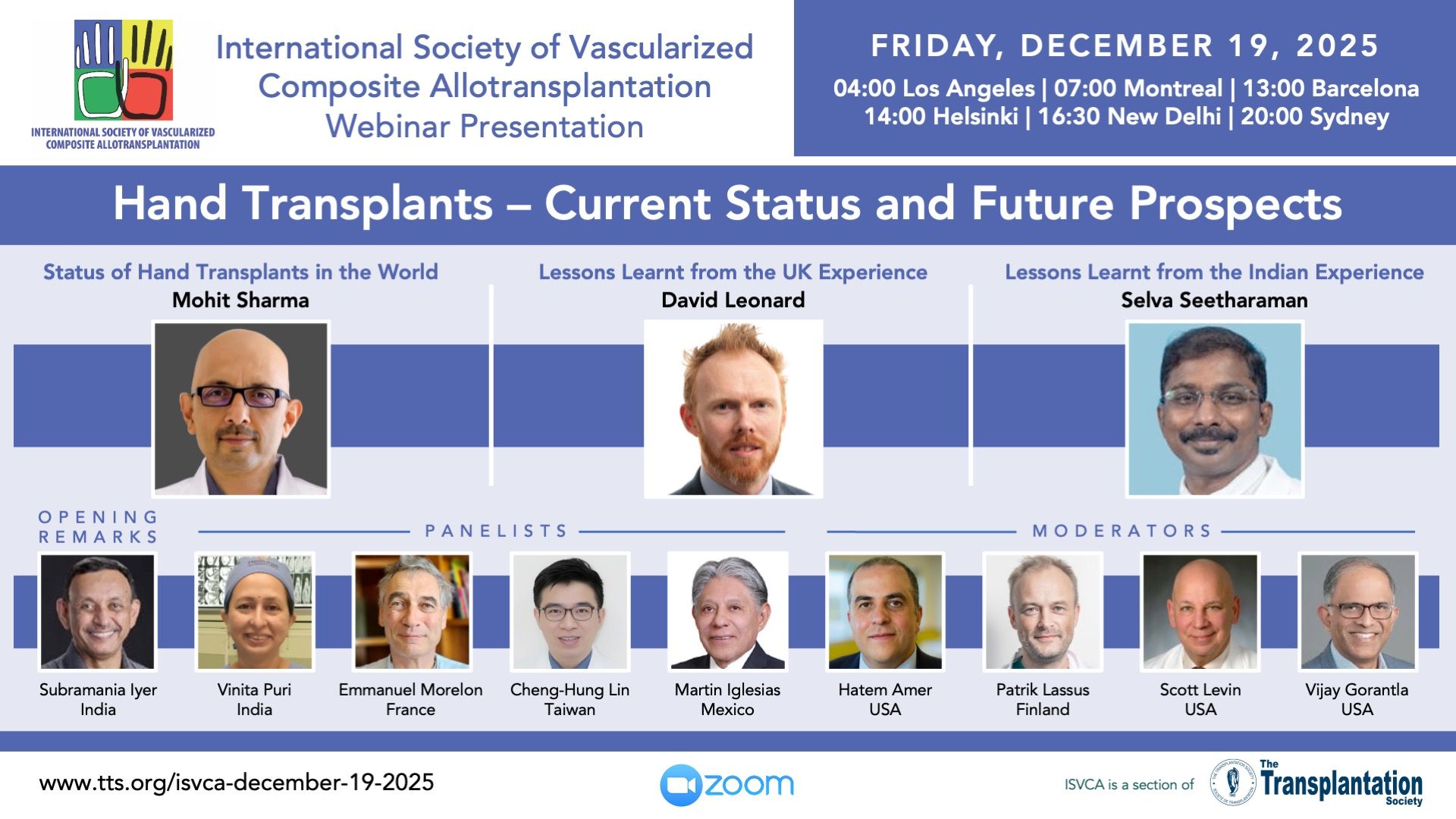
Upcoming Webinar Presentations
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!