TTS 2020 GOES VIRTUAL

A few weeks ago, we informed you that we would continue to monitor the evolution of the COVID-19 pandemic and government and institutional responses regarding travel restrictions so as to determine if a change to the format of TTS 2020 would become necessary.
As always, our primary concern is for the health and wellbeing of our delegates, exhibitors, staff and the wider community. We have therefore concluded that a physical meeting in Seoul in September 2020 would not be prudent.
Nevertheless, we strongly believe that an international congress of the transplant community is particularly important in 2020 in view of the unfortunate cancellation of many other international congresses.
We are therefore excited at the prospect of bringing you a virtual congress in September 2020 – one that will feature the most advanced scientific and clinical practices, as well as the most up-to-date developments on COVID-19. We will bring this in a forum that will enable delegates around the world to participate during their preferred time zones.
Over the coming weeks, we will bring you full details of this exciting new congress format.
JUST RELEASED - TRANSPLANTATION - JUNE ISSUE
This issue contains the important ILTS Consensus on Liver Transplant Oncology in four papers, which will help guide clinical practice in this increasingly relevant area. New approaches to increasing donor registration, analysis of the virtual crossmatch, paired kidney matching, and a number of papers will make us all reflect on approaches to immunosuppression even in the HLA identical kidney transplant. A couple of interesting experimental papers offer new strategies in lung transplantation and the storage of human corneas for three months may open a new era for corneal transplantation.
CLICK HERE TO ACCESS THIS ISSUE
TTS MEMBERS - CLICK HERE TO SIGN-IN FOR OPEN ACCESS THROUGH TTS.ORG
TRANSPLANTATION DIRECT - HIGHLIGHTED ARTICLE

Dr. Karen Keung, Editorial Fellow, Transplantation
Cognitive Improvement After Kidney Transplantation Is Associated With Structural and Functional Changes on MRI
van Sandwijk MS, ten Berge IJM, Caan MWA, et al.
Transplantation Direct: March 2020 - Volume 6 - Issue 3 - p e531
Few studies that have examined cognition changes following kidney transplantation have included controls or extensive neuroimaging. In this prospective observational cohort study, the authors evaluated the change in neurocognitive function after one year in 24 living transplant recipients compared with baseline, which was evaluated using the Amsterdam Neuropsychological Task (ANT) battery and verbal fluency tests. 24 kidney donors were included to control for learning effects, socioeconomic status, and surgery. MRI scans were performed in the recipients at baseline and at one- year post-transplant.
Both transplant donors and recipients had higher neuropsychologic testing scores at one year. Recipient scores were consistent with improved working memory capacity and attention, and these were significantly correlated with an increase in white matter volume and NA-acetylaspartate/creatine, a marker for neuronal integrity. These findings warrant further evaluation in larger prospective clinical cohorts with the inclusion of deceased donor transplant recipients.
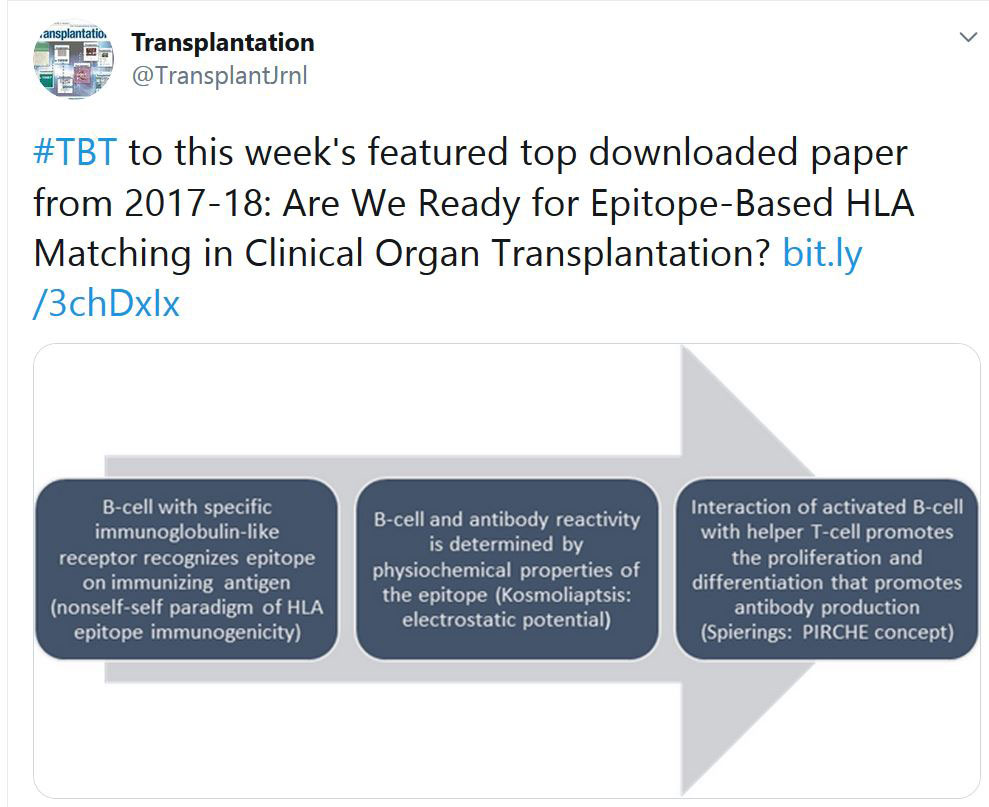
TRANSPLANTATION - WEEK'S MOST DOWNLOADED PAPER
TTS-ISVCA WEBINAR RECORDING IS AVAILABLE
TITLE: IMMUNOLOGY OF VCA
CLICK HERE TO VIEW THE RECORDING
(YOU MUST BE LOGGED INTO THE WEBSITE)
Webinar Summary:
To provide an overview on similarities and differences identified in VCA vs solid organ transplants.
A presentation of recent data from preclinical models characterizing cell populations that may protect grafts from strong immune responses directed towards skin containing VCA allografts.
Joint WIT-WHCOP Virtual Event: “#MeToo: Addressing Gender Inequity in Academic Medicine.”

 Please join the Women's Health Community of Practice (WHCOP) and the Women in Transplantation (WIT) for an exciting joint virtual event on Tuesday, June 2nd, 4:00 – 5:15 PM EDT.
Please join the Women's Health Community of Practice (WHCOP) and the Women in Transplantation (WIT) for an exciting joint virtual event on Tuesday, June 2nd, 4:00 – 5:15 PM EDT.
“#MeToo: Addressing Gender Inequity in Academic Medicine.”
Dr. Reshma Jagsi
Tuesday, June 2nd, 4:00 PM – 5:15 PM EDT
Guest speaker Dr. Reshma Jagsi and members of WHCOP and WIT will discuss the critical role of our memberships in supporting careers, research, and leadership for female transplant professionals.
The registration link will be posted closer to the event. If you are interested, please complete the form below and we will make sure that you are contacted with the Registration details.
Feel free to share this invitation with your colleagues. We hope to see you there!
«HOT OFF THE PRESS» RECENT PUBLICATIONS IDENTIFIED BY TTS EDUCATION COMMITTEE ON COVID-19
This week's selection made by: Enver Akalin, Millie Samaniego, Gabriel E. Gondolesi and Marlies Reinders

Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19.
Maximilian Ackermann, M.D., Stijn E. Verleden, Ph.D., Mark Kuehnel, Ph.D., Axel Haverich, M.D., Tobias Welte, M.D., Florian Laenger, M.D., Arno Vanstapel, Ph.D., Christopher Werlein, M.D., Helge Stark, Ph.D., Alexandar Tzankov, M.D., William W. Li, M.D., Vincent W. Li, M.D., Steven J. Mentzer, M.D., and Danny Jonigk, M.D.
DOI: 10.1056/NEJMoa2015432
This article examined lungs from 7 autopsies to try to better understand the pathophysiology of the progressive respiratory failure that leads death in Covid-19 infection. In Covid-19 patients the histological finding was a diffuse alveolar damage with perivascular T-cell infiltration, associated to severe endothelial, intracellular virus and disrupted cell membranes, together with widespread thrombosis and microangiopathy. Covid-19 patients had also intussusceptive angiogenesis, which it is a distinctive from equally severe influenza virus infection.
Remdesivir for the Treatment of Covid-19— Preliminary Report.
J.H. Beigel, K.M. Tomashek, L.E. Dodd, A.K. Mehta, B.S. Zingman, A.C. Kalil, E. Hohmann, H.Y. Chu, A. Luetkemeyer, S. Kline, D. Lopez de Castilla, R.W. Finberg, K. Dierberg, V. Tapson, L. Hsieh, T.F. Patterson, R. Paredes, D.A. Sweeney, W.R. Short, G. Touloumi, D.C. Lye, N. Ohmagari, M. Oh, G.M. Ruiz‑Palacios, T. Benfield, G. Fätkenheuer, M.G. Kortepeter, R.L. Atmar, C.B. Creech, J. Lundgren, A.G. Babiker, S. Pett, J.D. Neaton, T.H. Burgess, T. Bonnett, M. Green, M. Makowski, A. Osinusi, S. Nayak, and H.C. Lane, for the ACTT-1 Study Group Members*
DOI: 10.1056/NEJMoa2007764
This is a double-blind, randomized, placebo-controlled trial of intravenous remdesivir in adults hospitalized with Covid-19 with evidence of lower respiratory tract involvement. Patients were randomly assigned to receive either remdesivir (200 mg loading dose on day 1, followed by 100 mg daily for up to 9 additional days) or placebo for up to 10 days. Preliminaryresults from the 1059 patients (538 assigned to remdesivir and 521 to placebo) indicated that those who received remdesivir had a median recovery time of 11 days (95% confidence interval [CI],9 to 12), as compared with 15 days (95% CI, 13 to 19) in those who received placebo. The Kaplan- Meier estimates of mortality by 14 days were 7.1% with remdesivir and 11.9% with placebo (hazard ratio for death, 0.70; 95% CI, 0.47 to 1.04).
Mandeep R Mehra, Sapan S Desai, Frank Ruschitzka, Amit N Patel
Lancet. Published on lime May 22, 2020
https://doi.org/10.1016/ S0140-6736(20)31180-6
This article did a multinational registry analysis of the use of hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19. 96032 patients (mean age 53·8 years, 46·3% women) with COVID-19 were hospitalised during the study period and met the inclusion criteria. Of these, 14 888 patients were in the treatment groups (1868 received chloroquine, 3783 received chloroquine with a macrolide, 3016 received hydroxychloroquine, and 6221 received hydroxychloroquine with a macrolide) and 81 144 patients were in the control group. Each of these drug regimens was associated with decreased in-hospital survival and an increased frequency of ventricular arrhythmias when used for treatment of COVID-19.
Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19.
Daniel Blanco-Melo, Benjamin E. Nilsson-Payant, Wen-Chun Liu, Skyler Uhl, Daisy Hoagland, Rasmus Møller, Tristan X. Jordan, Kohei Oishi, Maryline Panis, David Sachs, Taia T. Wang, Robert E. Schwartz, Jean K. Lim, Randy A. Albrecht, and Benjamin R. tenOever
Cell 181, 1–10, May 28, 2020 ª 2020 Elsevier Inc.
https://doi.org/10.1016/j.cell.2020.04.026
This article documented an in-depth analysis of the transcriptional response to SARS-CoV-2 compared with other respiratory viruses. Cell and animal models of SARS-CoV-2 infection, in addition to transcriptional and serum profiling of COVID-19 patients, consistently revealed a unique and inappropriate inflammatory response. This response is defined by low levels of type I and III interferons juxtaposed to elevated chemokines and high expression of IL-6.
Alba Grifoni, Daniela Weiskopf, Sydney I. Ramirez, Jose Mateus, Jennifer M. Dan, Carolyn Rydyznski Moderbacher, Stephen A. Rawlings, Aaron Sutherland, Lakshmanane Premkumar, Ramesh S. Jadi, Daniel Marrama, Aravinda M. de Silva, April Frazier, Aaron Carlin, Jason A. Greenbaum,
CELL 11420
DOI: https://doi.org/10.1016/j.cell.2020.05.015
Using HLA class I and II predicted peptide ‘megapools’, circulating SARS-CoV-2-specific CD8+ and CD4+ T cells were identified in ~70% and 100% of COVID-19 convalescent patients, respectively. CD4+ T cell responses to spike, the main target of most vaccine efforts, were robust and correlated with the magnitude of the anti-SARS-CoV-2 IgG and IgA titers. The authors detected SARS-CoV-2-reactive CD4+ T cells in ~40-60% of
unexposed individuals, suggesting cross-reactive T cell recognition between circulating ‘common cold’ coronaviruses and SARS-CoV-2.
Feng-Cai Zhu, Yu-Hua Li, Xu-Hua Guan, Li-Hua Hou, Wen-Juan Wang, Jing-Xin Li, Shi-Po Wu, Bu-Sen Wang, Zhao Wang, Lei Wang, Si-Yue Jia, Hu-Dachuan Jiang, Ling Wang, Tao Jiang, Yi Hu, Jin-Bo Gou, Sha-Bei Xu, Jun-Jie Xu, Xue-Wen Wang, Wei Wang, Wei Chen
Lancet May 2020
https://doi.org/10.1016/ S0140-6736(20)31208-3
This is a dose-escalation, single-centre, open-label, non-randomised, phase 1 trial of an Ad5 vectored COVID-19 vaccine in Wuhan, China. 108 participants (51% male, 49% female; mean age 36·3 years) were recruited and received the low dose (n=36), middle dose (n=36), or high dose (n=36) of the vaccine. ELISA antibodies and neutralising antibodies increased significantly at day 14, and peaked 28 days post-vaccination. Specific T-cell response peaked at day 14 post-vaccination.
CORONAVIRUS (COVID-19) UPDATE DASHBOARD
The Transplantation Society (TTS) and our journal Transplantation have developed online resources to keep you informed on the Coronavirus (COVID-19) outbreak.
- TTS Coronavirus (COVID-19) Dashboard
www.tts.org/covid-19 - Transplantation Global Transplantation COVID Report
www.tts.org/txjcovid19
We are also requesting contributions and news from the transplant community to be sent to covid-19@tts.org for inclusion on our resources page.
In this dashboard, you will find links to TTS and other global and regional resources, as well as interactive maps, publications and webinars. We encourage you to explore this dashboard and share with your colleagues.
Website - www.tts.org/covid-19
Editors and contributors to Transplantation have shared their thoughts on how they are dealing with the current crisis. While we understand that the information of today may be quite different tomorrow in this fast-moving pandemic, this report will open our forum of an international exchange on COVID for the transplant community.
Website - www.tts.org/txjcovid19
Please send your own contributions and news to covid-19@tts.org for inclusion on our resources page.
IN THE NEWS
CHARACTERISTICS AND OUTCOMES OF RECIPIENTS OF HEART TRANSPLANT WITH CORONAVIRUS DISEASE 2019
May 13 - In this case series of 28 patients who had received heart transplant in a large academic center, the case fatality rate among patients infected with COVID-19 was 25%. Cardiovascular comorbidities were frequent in this population, and immunosuppressive therapy was reduced in most patients.
FOLLOWING-UP ALLOGENEIC TRANSPLANTATION RECIPIENTS DURING THE COVID-19 PANDEMIC
May 22 - After allogeneic transplantation, comprehensive clinical follow-up is recommended for early detection of post-transplantation infections and other common complications such as endocrine disease or metabolic syndrome, and to ensure patients can enjoy quality of life. The pandemic of coronavirus disease 2019 (COVID-19) started spreading around the world in Feb 2020, presenting an unexpected challenge for transplantation services, including clinical follow-up.
CLINICAL DISTANCING OF PATIENTS WITH ADVANCED HF, TRANSPLANT MAY PROTECT AGAINST COVID-19
May 22 - One hospital system protected its patients with advanced HF and cardiac transplant from COVID-19 by safely moving them from a central hospital, which was redesigned to care for patients with COVID-19, to a cardiac specialty hospital with no patients with COVID-19, according to a paper published in The Journal of Heart and Lung Transplantation.
KIDNEY TRANSPLANTS: BETTER RESULTS CAN BE INFERRED FROM A LARGER NUMBER OF CASES
May 22 - In complex operations, is there a correlation between the volume of service provided per hospital and the quality of the treatment outcome? This question is addressed in eight commissions on minimum volumes that the Federal Joint Committee (G-BA) has issued to the Institute for Quality and Efficiency in Health Care (IQWiG). The IQWiG report is now available for the fifth intervention to be tested, kidney transplantation.
DO MEN GET PRIORITY FOR LIVER TRANSPLANT?

May 21 - New evidence for sex disparity prompts call for "concrete changes" in allocation policies.
UPCOMING MEETINGS AND ANNOUNCEMENTS
11th International Pediatric Intestinal Failure and Rehabilitation Symposium
International Transplantation Science Meeting
ITS 2021 meeting in the San Diego area — October 17-20, 2021

After careful consideration, and in light of the ongoing Coronavirus developments, we’ve made the difficult decision to postpone the ITS 2020 Meeting in Europe to 2022. Stay tuned for dates and location.
We would like to take this opportunity to confirm that we will have the 2021 ITS meeting in the San Diego area from October 17-20, 2021.
Further details will be available this summer.
We look forward to hosting you in California next year.
Until then, stay safe and healthy!
3rd IPITA/JDRF/HSCI Conference on Stem Cell Derived Beta Cells
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!