SERVING THE INTERNATIONAL TRANSPLANTATION COMMUNITY
As we wish to bring our Congress to the entire international transplantation community, and in everyone’s individual time zone, we developed a program and schedule that would allow all delegates to access the Congress programming during two time periods regardless of geographic region.
JUST RELEASED - TRANSPLANTATION - JULY ISSUE
This month has an 'allocation bias,' examining many factors that can lead to bias in allocation from the bias in laboratory measurement of creatinine in MELD through to center volumes in access to lung transplants and social, ethnic, and racial biases in kidney transplantation. The rise of the non-HLA antibody is described in the Visual Abstract on the cover page and in a fascinating paper while posttransplant monoclonal immunoglobulin associated nephropathy is nicely reviewed. The conundrum of maintaining protection from microbial pathogens while inducing tolerance is carefully dissected in an expert review. For coronavirus you must see our COVID-19 Map, where all the real-time updates are available.
CLICK HERE TO ACCESS THIS ISSUE
TTS MEMBERS - CLICK HERE TO SIGN-IN FOR OPEN ACCESS THROUGH TTS.ORG
TRANSPLANTATION - HIGHLIGHTED ARTICLE

Dr. Jeremy R. Chapman, Editor-in-Chief, Transplantation
Immunosuppression and Graft Rejection in Living related HLA-identical Renal Transplantation: The RADOVFULL Study
Ossman R, Jamme M, Moulin B, et al.
Transplantation: June 2020 - Volume 104 - Issue 6 - p 1256-1262
This is a French national study of outcomes for HLA identical siblings with transplants undertaken between 2002 and 2012. In that period 27,218 transplants were performed, of which 2475 were from living elated donors. HLA Identical siblings donated in 163 cases of whom 21 had a rejection episode or a rate of 12.9% acute rejection. Perhaps that is what we might expect – or a little higher than we might hope for from HLA identical sibling transplants, but nothing extraordinary to modify our clinical approach. You need to read a little deeper and a fact leaps out of the page that the average time to first acute rejection was 24 months. Clearly there is something in the way that we manage these patients that is leading to a very different profile of acute rejection compared to the non identical living related donor. Multivariate analysis reveals two significant risk factors – younger age and minimisation of immunosuppression. Perhaps these two issues are related - with the young given official licence to minimize their medications do so with a determination not shown when the call was compliance with medication! Perhaps we need to be more careful in the way in which we discuss HLA identity with the patients, giving unwitting licence to our patients to cease immunosuppressive medication when we know full well that this can lead to graft rejection of HLA identical grafts? A good study that is worthy of your careful attention.
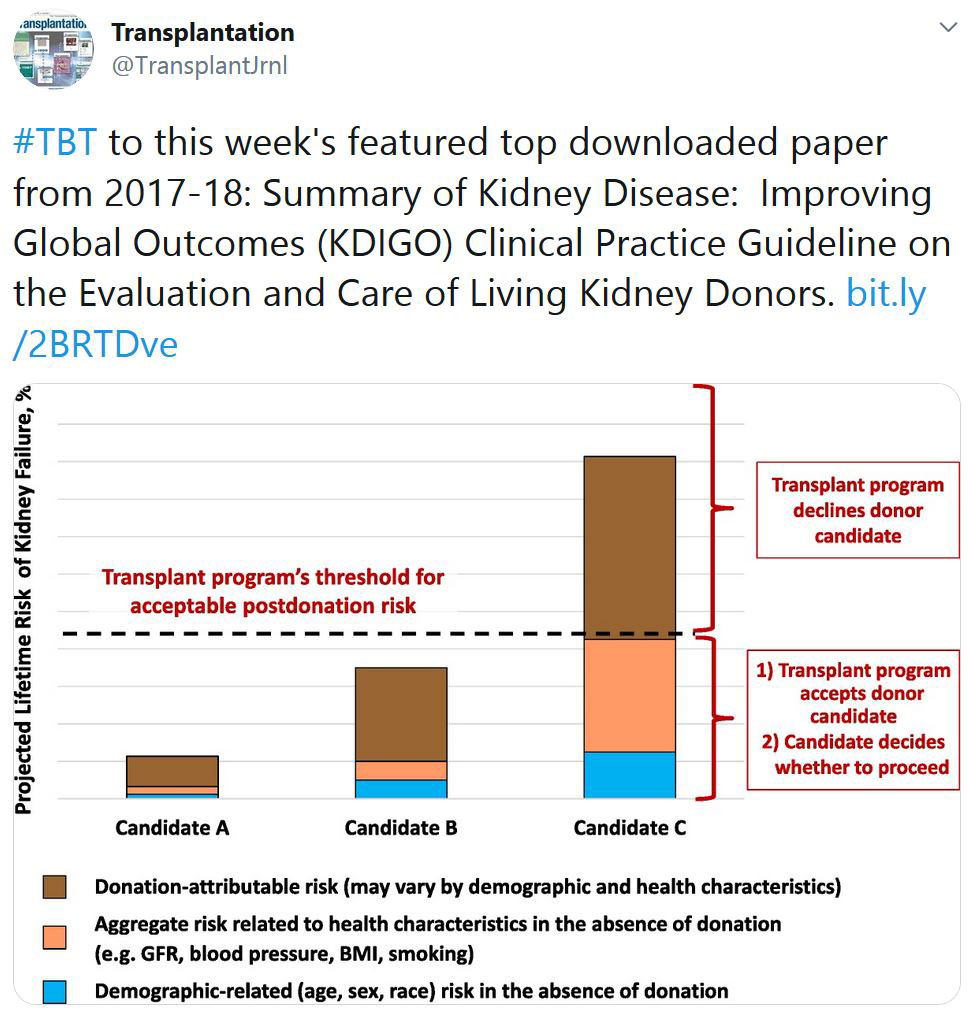
TRANSPLANTATION - WEEK'S MOST DOWNLOADED PAPER
ISN-TTS WEBINAR RECORDING NOW AVAILABLE
«HOT OFF THE PRESS» RECENT PUBLICATIONS IDENTIFIED BY TTS EDUCATION COMMITTEE ON COVID-19
This week's selection made by: Enver Akalin Marcelo Cantarovich and Manisha Sahay

Federico Alberici, Elisa Delbarba, Chiara Manenti, Laura Econimo, Francesca Valerio, Alessandra Pola, Camilla Maffei, Stefano Possenti, Bernardo Lucca, Roberta Cortinovis, Vincenzo Terlizzi, Mattia Zappa, Chiara Saccà, Elena Pezzini, Eleonora Calcaterra, Paola Piarulli, Alice Guerini, Francesca Boni, Agnese Gallico, Alberto Mucchetti, Stefania Affatato, Sergio Bove, Martina Bracchi, Ester Maria Costantino, Roberto Zubani, Corrado Camerini, Paola Gaggia, Ezio Movilli, Nicola Bossini, Mario Gaggiotti, Francesco Scolari
Kidney International (2020) 98, 20–26; https://doi.org/10.1016/j.kint.2020.04.030
During March 2020, within an overall population of 643 hemodialysis patients, SARS-CoV-2 RNA positivity was detected in 94 (15%). At disease diagnosis, 37 of the 94 (39%) patients(group 1) were managed on an outpatient basis, whereas the remaining 57 (61%) (group 2) required hospitalization. In group 1 8% died and 5% developed acute respiratory distress syndrome (ARDS). In group 2, Forty two percent died and 79% developed ARDS. Overall mortality rate for the entire cohort was 29%.
Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections.
Quan-Xin Long, Xiao-Jun Tang, Qiu-Lin Shi, Qin Li, Hai-Jun Deng , Jun Yuan, Jie-Li Hu, Wei Xu, Yong Zhang, Fa-Jin Lv, Kun Su, Fan Zhang, Jiang Gong, Bo Wu, Xia-Mao Liu,Jin-Jing Li, Jing-Fu Qiu, Juan Chen and Ai-Long Huang
Nature Medicine. https://doi.org/10.1038/s41591-020-0965-6
This article studied 37 asymptomatic individuals who were diagnosed with RT–PCR-confirmed SARS-CoV- infections but without any relevant clinical symptoms in the preceding 14 d and during hospitalization. The median duration of viral shedding in the asymptomatic group was 19 d (interquartile range (IQR), 15–26 d). The asymptomatic group had a significantly longer duration of viral shedding than the symptomatic group (log-rank P = 0.028). The virus-specific IgG levels in the asymptomatic group were significantly lower (P = 0.005) relative to the symptomatic group in the acute phase. Of asymptomatic individuals, 93.3% (28/30) and 81.1% (30/37) had reduction in IgG and neutralizing antibody levels, respectively, during the early convalescent phase, as compared to 96.8% (30/31) and 62.2% (23/37) of symptomatic patients. Forty percent of asymptomatic individuals became seronegative and 12.9% of the symptomatic group became negative for IgG in the early convalescent phase. These data suggest that asymptomatic individuals had a weaker immune response to SARS-CoV-2 infection.
Tocilizumab in patients with severe COVID-19: a retrospective cohort study.
Giovanni Guaraldi, Marianna Meschiari, Alessandro Cozzi-Lepri, Jovana Milic, Roberto Tonelli, Marianna Menozzi, Erica Franceschini, Gianluca Cuomo, Gabriella Orlando, Vanni Borghi, Antonella Santoro, Margherita Di Gaetano, Cinzia Puzzolante, Federica Carli, Andrea Bedini, Luca Corradi, Riccardo Fantini, Ivana Castaniere, Luca Tabbì, Massimo Girardis, Sara Tedeschi, Maddalena Giannella, Michele Bartoletti, Renato Pascale, Giovanni Dolci, Lucio Brugioni, Antonello Pietrangelo, Andrea Cossarizza, Federico Pea, Enrico Clini, Carlo Salvarani, Marco Massari, Pier Luigi Viale, Cristina Mussini
The Lancet Rheumatology. June 24, 2020; https://doi.org/10.1016/S2665-9913(20)30173-9
This retrospective, observational cohort study included adults (≥18 years) with severe COVID-19 pneumonia who were admitted to tertiary care centres. Of 1351 patients admitted, 544 (40%) had severe COVID-19 pneumonia. 57 (16%) of 365 patients in the standard care group needed mechanical ventilation, compared with 33 (18%) of 179 patients treated with tocilizumab (p=0·41; 16 [18%] of 88 patients treated intravenously and 17 [19%] of 91 patients treated subcutaneously). 73 (20%) patients in the standard care group died, compared with 13 (7%; p<0·0001) patients treated with tocilizumab. After adjustment for sex, age, recruiting centre, duration of symptoms, and SOFA score, tocilizumab treatment was associated with a reduced risk of invasive mechanical ventilation or death (adjusted hazard ratio 0·61, 95% CI 0·40–0·92; p=0·020). 24 (13%) of 179 patients treated with tocilizumab were diagnosed with new infections, versus 14 (4%) of 365 patients treated with standard of care alone (p<0·0001).
CORONAVIRUS (COVID-19) UPDATE DASHBOARD
The Transplantation Society (TTS) and our journal Transplantation have developed online resources to keep you informed on the Coronavirus (COVID-19) outbreak.
- TTS Coronavirus (COVID-19) Dashboard
www.tts.org/covid-19 - Transplantation Global Transplantation COVID Report
www.tts.org/txjcovid19
We are also requesting contributions and news from the transplant community to be sent to covid-19@tts.org for inclusion on our resources page.
In this dashboard, you will find links to TTS and other global and regional resources, as well as interactive maps, publications and webinars. We encourage you to explore this dashboard and share with your colleagues.
Website - www.tts.org/covid-19
Editors and contributors to Transplantation have shared their thoughts on how they are dealing with the current crisis. While we understand that the information of today may be quite different tomorrow in this fast-moving pandemic, this report will open our forum of an international exchange on COVID for the transplant community.
Website - www.tts.org/txjcovid19
Please send your own contributions and news to covid-19@tts.org for inclusion on our resources page.
IN THE NEWS
STUDY FINDS HCV-POSITIVE LIVERS SAFE FOR TRANSPLANTATION; HEPATITIS C CAN BE CURED AFTERWARD
June 23 - Patients who received a transplanted liver infected with hepatitis C and were later treated for the infection performed as well in recovery as transplant patients who received an organ free of infection, according to study from the University of Cincinnati College of Medicine and UC Health recently published in the journal Liver Transplantation.
FECAL MICROBIOTA TRANSPLANT MAY EMERGE AS A TREATMENT STRATEGY IN MELANOMA FOLLOWING IMMUNOTHERAPY FAILURE
June 27 - n a phase 1 trial of patients with advanced melanoma whose disease progressed on immunotherapy, objective responses were achieved with the use of fecal microbiota transplant (FMT) in 30% of those who were re-challenged with a PD-1 inhibitor (NCT03353402).
DEFECT IN PANCREAS ALPHA CELLS LINKED TO DIABETES, STANFORD MEDICINE STUDY SHOWS
June 24 - Pancreatic alpha cells from people with diabetes release excess amounts of glucagon, a hormone important in blood sugar control, in a new Stanford-developed mouse model of transplanted human islets.
ANTIVIRAL DRUGS HELP PREVENT HCV INFECTIONS IN TRANSPLANT RECIPIENTS
June 26 - An antiviral regimen could allow hepatitis C virus (HCV) organ donors to safely transplant organs into non-infected recipients. A team, led by Jordan J. Field, MD, Toronto General Hospital, examined whether antiviral drugs combined with an HCV entry blocker given before and for 7 days following an organ transplant would safety reduce the likelihood of an HCV infection in organ recipients from HCV-infected donors.
LAB-GROWN MINI-ORGANS REVEAL THE DAMAGE INFLICTED BY COVID-19
June 27 - As part of global studies on the coronavirus, organoids help scientists understand the effects of the virus and how to treat them.
UPCOMING MEETINGS AND ANNOUNCEMENTS
18th Asian Pacific Congress of Nephrology (APCN)
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!