NEJM July 17,2020; DOI: 10.1056/NEJMoa2021436
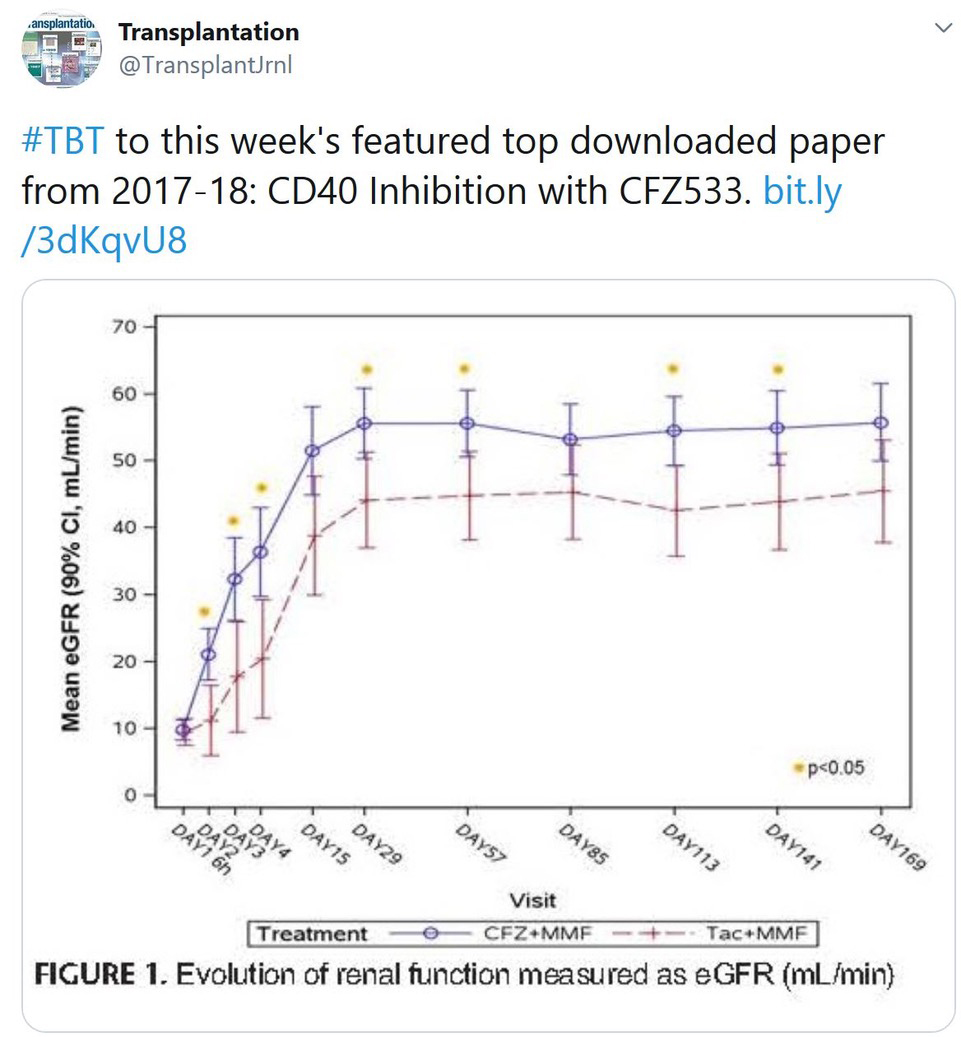
This is a controlled, open-label trial comparing COVID patients to receive oral or intravenous dexamethasone (at a dose of 6 mg once daily) for up to 10 days or to receive usual care alone. A total of 2104 patients were assigned to receive dexamethasone and 4321 to receive usual care. Overall, 482 patients (22.9%) in the dexamethasone group and 1110 patients (25.7%) in the usual care group died within 28 days after randomization(age-adjusted rate ratio, 0.83; 95% confidence interval [CI], 0.75 to 0.93; P<0.001). In the dexamethasone group, the incidence of death was lower than that in the usual care group among patients receiving invasive mechanical ventilation (29.3% vs. 41.4%; rate ratio, 0.64; 95% CI, 0.51 to 0.81) and among those receiving oxygen without invasive mechanical ventilation(23.3% vs. 26.2%; rate ratio, 0.82; 95% CI, 0.72 to 0.94) but not among those who were receiving no respiratory support at randomization (17.8% vs. 14.0%; rate ratio,1.19; 95% CI, 0.91 to 1.55).
L.A. Jackson, E.J. Anderson, N.G. Rouphael, P.C. Roberts, M. Makhene, R.N. Coler, M.P. McCullough, J.D. Chappell, M.R. Denison, L.J. Stevens, A.J. Pruijssers, A. McDermott, B. Flach, N.A. Doria‑Rose, K.S. Corbett, K.M. Morabito, S. O’Dell, S.D. Schmidt, P.A. Swanson II, M. Padilla, J.R. Mascola, K.M. Neuzil, H. Bennett, W. Sun, E. Peters, M. Makowski, J. Albert, K. Cross, W. Buchanan, R. Pikaart‑Tautges, J.E. Ledgerwood, B.S. Graham, and J.H. Beigel, for the mRNA-1273 Study Group*
NEJM July 14, 2020, DOI: 10.1056/NEJMoa2022483
This is a phase 1, dose-escalation, open-label trial including 45 healthy adults, 18 to 55 years of age, who received two vaccinations, 28 days apart, with mRNA-1273 in a dose of 25 μg, 100 μg, or 250 μg. There were 15 participants in each dose group. The mRNA-1273 vaccine induced anti–SARS-CoV-2 immune responses in all participants, and no trial-limiting safety concerns were identified. These findings support further development of this vaccine.