TTS 2020 Virtual Congress - A Virtual Learning Experience
A big thank you to all attendees and supporters of the TTS 2020 Virtual Congress. Presentation recordings are now available for all TTS members and congress attendees. A personalized Certificate of Participation is available to all registered attendees and can be accessed directly from your profile through the WebApp.
TTS 2020 Virtual Congress - Reminder
September 24 - Post-Congress Symposium
Questions & Answers Period + Live Discussions
Session 1: 9-10:30 AM EDT (New York)
Other local start times: 6 AM PDT (San Francisco) / 15h CET (Frankfurt) / 23h Sydney / 22h Seoul
Session 2: 7-8:30 PM EDT (New York)
Other local start times: 4 PM PDT (San Francisco) / Sept 25 - 1h CET (Frankfurt) / 9h Sydney / 8h Seoul

TRANSPLANTATION DIRECT - HIGHLIGHTED ARTICLE
Dr. Jeremy R. Chapman, Editor-in-Chief, Transplantation
Urinary Cell Transcriptome Profiling and Identification of ITM2A, SLAMF6, and IKZF3 as Biomarkers of Acute Rejection in Human Kidney Allograft
Dooley BJ, Verma A, Ding R, et al.
Transplantation Direct: August 2020 - Volume 6 - Issue 8 - p e588
This well-known research team performed RNA sequencing and bioinformatics of genome-wide transcriptome profiles of urinary cells from kidney transplant recipients. Their aim was to identify novel mRNAs potentially relevant to both T cell and antibody mediated rejection. Customized RT-QPCR assays were then used to validate their abundance in urinary cells in 22 biopsy proven T cell mediated rejections and 7 antibody mediated samples, compared to 24 samples taken at times of biopsies not demonstrating rejection. They found 127 genes shared between TCMR and AMR and tested 3 novel mRNAs—ITM2A, SLAMF6, and IKZF3—for absolute quantification and validation by customized RT-QPCR assays. The abundance of all 3 mRNAs was significantly higher in urine from rejecting than non-rejecting samples of urine but were similar in both T cell and Antibody mediated rejection.
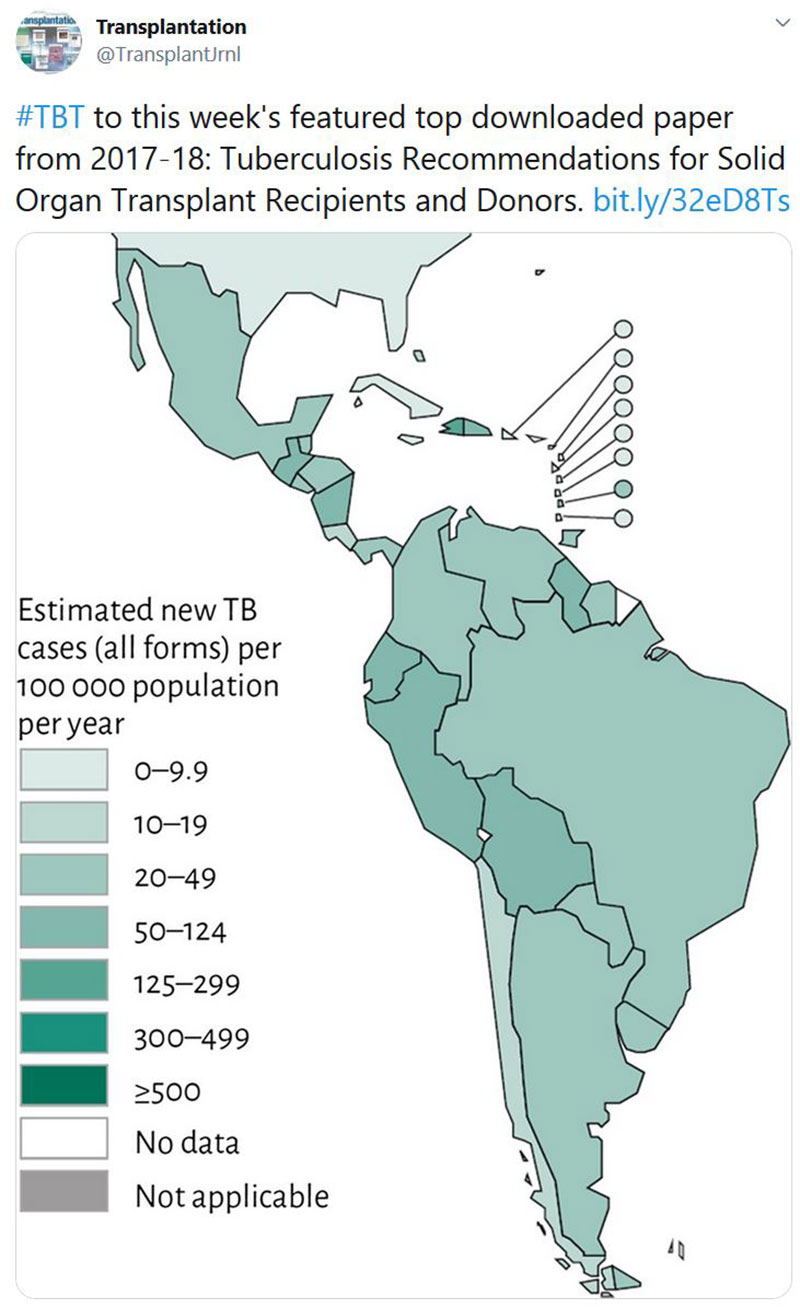
TRANSPLANTATION - WEEK'S MOST DOWNLOADED PAPER
«HOT OFF THE PRESS»
RECENT PUBLICATIONS IDENTIFIED BY TTS EDUCATION COMMITTEE ON COVID-19
Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19
Peng, Y., Mentzer, A.J., Liu, G. et al.
Nat Immunol (2020). Published: September 4, 2020. https://doi.org/10.1038/s41590-020-0782-6
This article studied T cell memory in 42 patients following recovery from COVID-19 (28 with mild disease and 14 with severe disease) and 16 unexposed donors, using interferon-γ-based assays with peptides spanning SARS-CoV-2 except ORF1. The breadth and magnitude of T cell responses were significantly higher in severe as compared with mild cases. Total and spike-specific T cell responses correlated with spike-specific antibody responses. The authors identified 41 peptides containing CD4+ and/or CD8+ epitopes, including six immunodominant regions. Six optimized CD8+ epitopes were defined, with peptide–MHC pentamer-positive cells displaying the central and effector memory phenotype. In mild cases, higher proportions of SARS-CoV-2-specific CD8+ T cells were observed. The identification of T cell responses associated with milder disease will support an understanding of protective immunity and highlights the potential of including non-spike proteins within future COVID-19 vaccine design.
Robust T cell response towards spike, membrane, and nucleocapsid SARS-CoV-2 proteins is not associated with recovery in critical COVID-19 patients
Constantin J. Thieme et al.
Cell Reports Medicine, DOI: https://doi.org/10.1016/j.xcrm.2020.100092
This study compares the SARS-CoV-2-reactive T cell responses in moderate, severe, and critical COVID-19 patients and unexposed donors. Overlapping peptide pools of all three proteins induce SARS-CoV-2-reactive T cell response with dominance of CD4+ over CD8+ T cells and demonstrate interindividual immunity against the three proteins. M-protein induces the highest frequencies of CD4+ T cells, suggesting its relevance for diagnosis and vaccination. Importantly, T cell response of critical COVID-19 patients is robust and comparable or even superior to non-critical patients. Virus clearance and COVID-19 survival are not associated with either SARS-CoV-2 T cell kinetics or magnitude of T cell responses, respectively. Thus, this data disprove the hypothesis of insufficient SARS-CoV-2-reactive immunity in critical COVID-19. Conversely, it indicates that activation of differentiated memory effector T cells could cause hyper-reactivity and immunopathogenesis in critical patients.
Effect of Recombinant Human Granulocyte Colony–Stimulating Factor for Patients With Coronavirus Disease 2019 (COVID-19) and Lymphopenia: A Randomized Clinical Trial
Cheng L, Guan W, Duan C, et al.
JAMA Intern Med. Published online September 10, 2020. doi:10.1001/jamainternmed.2020.5503
Lymphopenia is common and correlates with poor clinical outcomes in patients with coronavirus disease 2019 (COVID-19).This is an open-label, multicenter, randomized clinical trial at 3 participating centers in China. The main eligibility criteria were pneumonia, a blood lymphocyte cell count of 800 per or lower, and no comorbidities. Time to clinical improvement was similar between groups (rhG-CSF group median of 12 days (IQR, 10-16 days) vs usual care group median of 13 days (IQR, 11-17 days); hazard ratio, 1.28; 95% CI, 0.95-1.71; P=.06). The proportion of patients progressing to acute respiratory distress syndrome, sepsis, or septic shock was lower in the rhG-CSF group (rhG-CSF group, 2% vs usual care group, 15%; difference, −13%; 95%CI, −21.4% to −5.4%). At 21 days, 2 patients (2%) had died in the rhG-CSF group compared with 10 patients (10%) in the usual care group (hazard ratio, 0.19; 95%CI, 0.04-0.88). In preliminary findings from a randomized clinical trial, rhG-CSF treatment for patients with COVID-19 with lymphopenia but no comorbidities did not accelerate clinical improvement, but the number of patients developing critical illness or dying may have been reduced.
CORONAVIRUS (COVID-19) UPDATES
INVITATION TO PARTICIPATE

Dear Members,
Researchers at McGill University are currently conducting a study entitled LIST-COVID-19 to study the impact of the COVID-19 pandemic on transplantation activity, volumes and immunosuppression practices on a global scale. The study will also help better understand the current and anticipated risks to transplantation during the pandemic and inform clinical practice during the “ramp-up” phase. The Transplantation Society is supporting the project, as well as several National and International Societies.
They are looking for physicians who take care of patients with a solid organ transplant to take a 10-15 minute survey on behalf of their center and program. Please note this is not a patient registry and no specific patient-level data is being collected. Please be assured they will only perform a collective analysis of the survey questions and no personal information identifying the participants will be divulged. The survey responses will be confidential. If you are interested in participating, please contact them at the emails listed below.
Investigators: Shaifali Sandal, MD and Marcelo Cantarovich, MD
Contact: shaifali.sandal@mcgill.ca and marcelo.cantarovich@muhc.mcgill.ca
The Transplantation Society (TTS) and our journal Transplantation have developed online resources to keep you informed on the Coronavirus (COVID-19) outbreak.
- TTS Coronavirus (COVID-19) Dashboard
www.tts.org/covid-19 - Transplantation Global Transplantation COVID Report
www.tts.org/txjcovid19
We are also requesting contributions and news from the transplant community to be sent to covid-19@tts.org for inclusion on our resources page.
In this dashboard, you will find links to TTS and other global and regional resources, as well as interactive maps, publications and webinars. We encourage you to explore this dashboard and share with your colleagues.
Editors and contributors to Transplantation have shared their thoughts on how they are dealing with the current crisis. While we understand that the information of today may be quite different tomorrow in this fast-moving pandemic, this report will open our forum of an international exchange on COVID for the transplant community.
Website - www.tts.org/txjcovid19
Please send your own contributions and news to covid-19@tts.org for inclusion on our resources page.
IN THE NEWS
OUTCOMES OF TRANSPLANTATION WITH KIDNEY FROM DECEASED DONOR WITH ADPKD
Sept. 16 - Patients with autosomal dominant polycystic kidney disease (ADPKD) are routinely not considered as potential donors for renal transplantation. There is a concern that peri-transplant acute kidney injury may accelerate cystogenesis in kidneys from patients with ADPKD similarly as it accelerates cystogenesis in animal ADPKD models. However, the immunosuppression may have cystogenesis-inhibiting effects, as has been seen in animal ADPKD models.
COVID-19 HAS TAKEN A TOLL ON ORGAN DONATION
Sept. 17 - Transplants of organs from dead donors haven't slowed during the coronavirus pandemic, but living donor transplants remain suspended in many places, an expert says.
DONOR OBESITY MAY NOT AFFECT OUTCOMES IN HEART TRANSPLANTATION
Sept. 16 - Transplant recipients of hearts from donors with severe obesity experienced no more adverse events and similar 1-year survival as those who received hearts from donors without severe obesity, according to researchers.
RESEARCHERS FIND 'CELLULAR COMPASS' GUIDES STEM CELL DIVISION IN PLANTS
Sept. 17 - Biologists observing the formation of leaves noticed the nuclei moved in bewildering ways. Further investigation uncovered proteins that act as compasses and motors, guiding the divisions of individual cells to create the overall pattern of the leaf.
UPCOMING MEETINGS AND ANNOUNCEMENTS
SPLIT Virtual Annual Meeting
18th Asian Pacific Congress of Nephrology (APCN)
IPITA-IXA-CTRMS Joint Congress
FOCIS 2020 Virtual Annual Meeting
TTS members are invited to attend the FOCIS 2020 Virtual Annual Meeting – THE meeting in translational immunology!
The Federation of Clinical Immunology Societies exists to improve human health through immunology.
The FOCIS 2020 Virtual Annual Meeting on October 28-31, 2020, brings together an interdisciplinary group of world-renowned physicians and researchers to share the latest findings on diseases impacting the immune system.
Asian Transplantation Week (ATW) 2020
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!