Upcoming Webinars

New Date!
ISN-TTS JOINT WEBINAR
Donor/Recipient Pair:
RISKS VS. GAINS - KIDNEY FUNCTION
Open to all healthcare professionals
Speaker: Marcelo Cantarovich, TTS President
Moderator: Jean Tchervenkov, Montreal, Canada
Free to attend
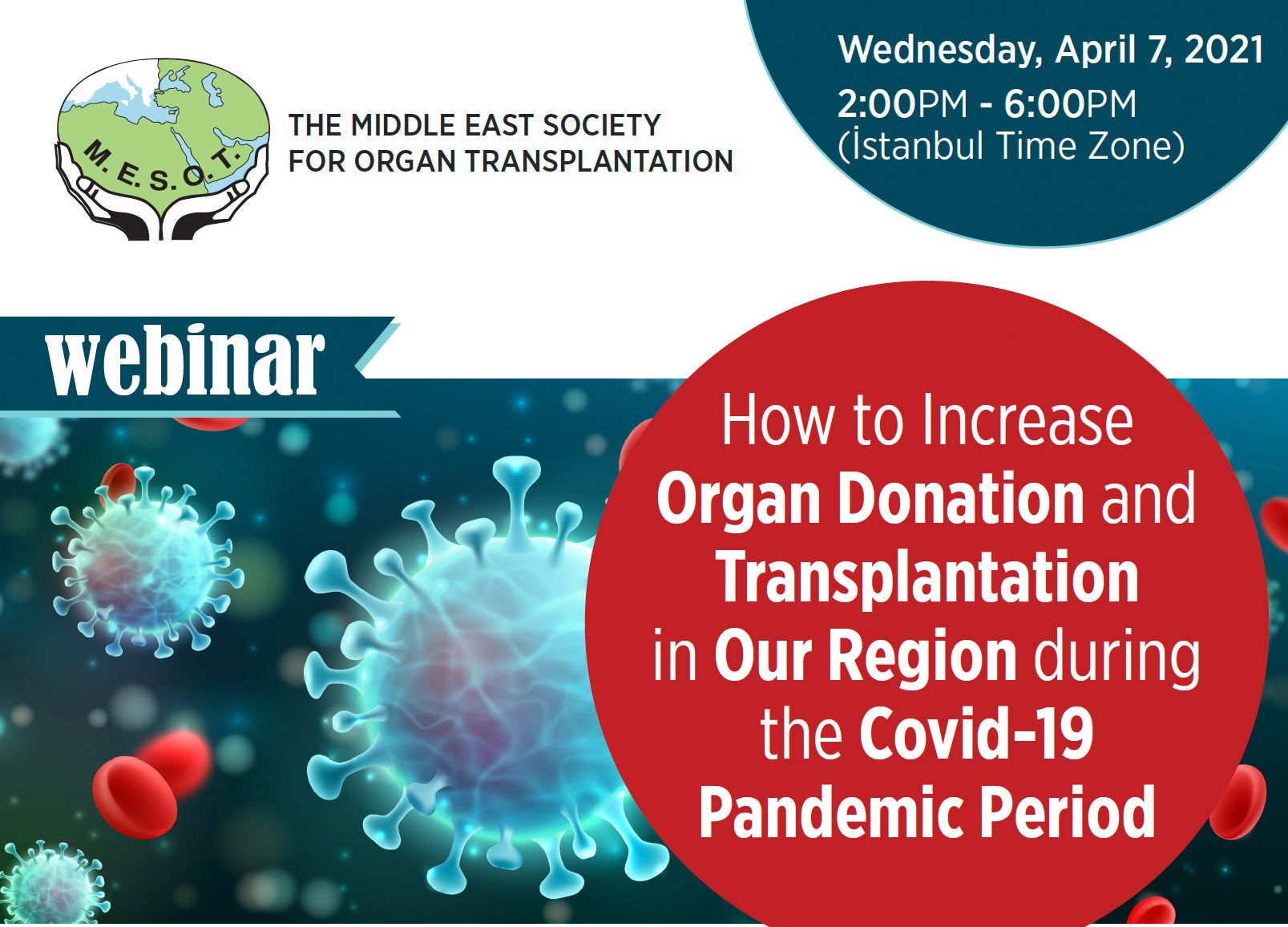
The Middle East Society For Organ Transplantation (MESOT) invites you to a new webinar on How to Increase Organ Donation and Transplantation in Our Region during the Covid-19 Pandemic Period.
With COVID-19 pandemic spreading across the globe since last year, it had the immediate effect of severely reducing living and deceased organ donation and transplantation activity worldwide.
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!












