IXA Update - November 2017
IXA 2019 CONGRESS


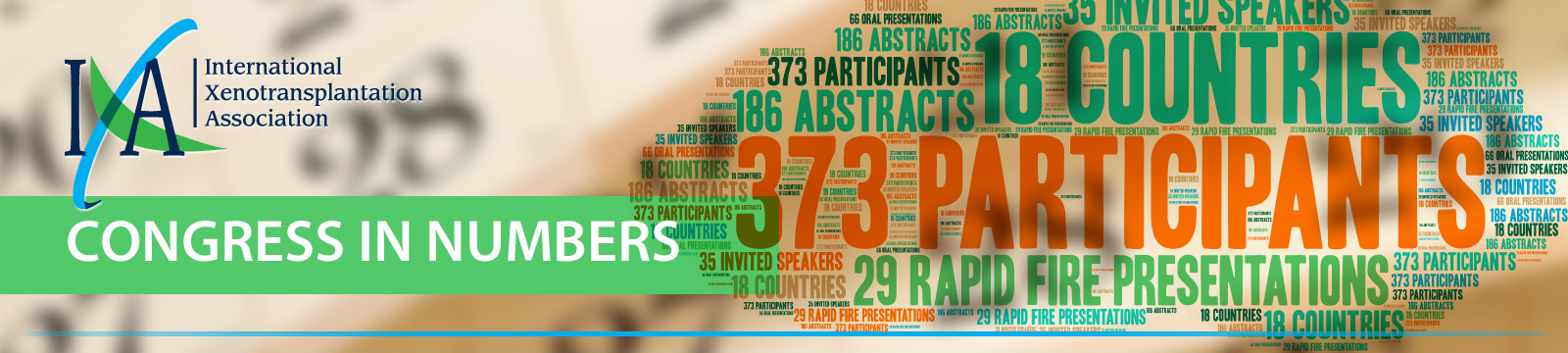
IXA 2017 Congress
This year marks the 20th anniversary of the founding of the International Xenotransplantation Association. Our 14th biennial Congress, recently held in Baltimore, Maryland, demonstrated the growth and energy within the Association. The meeting brought together a dynamic, influential group of scientists and clinicians whose work will help to set the future of new clinical treatments with the potential to transform modern medicine.
Satellite meetings designed to foster discussion on hot topics saw the participation of a broader audience. The Hyperimmunized/iABO Symposium brought together clinicians and surgeons who are active in the management of sensitized patients as well as those conducting related cutting-edge clinical trials. The Swine in Biomedical Research Conference focused on scientific as well as organizational aspects of generating swine and advancing relevant animal models for biomedical applications, with the participation of entrepreneurs and industry partners.
The FDA/IXA Symposium continued the ongoing dialogue between the xenotransplantation scientific community and the agencies that oversee the regulatory aspects of medical therapies in US. Recent scientific advances and challenges of risk/benefit were discussed in a positive and constructive way. The IXA-Theological Symposium took place to address ethical issues on xenotransplantation from the prospective of three religions (Christianity, Judaism, and Islam). Scientists and clinicians had the opportunity to interact with theologians on the moral duties and responsibilities of conducting novel biomedical research. The ethical groundwork discussed here, no less than the regulatory meetings with the FDA, will become even more prominent as we develop strategies and models that bring xenotransplantation closer to the clinic.
Participation in the combined meetings by these clinicians, scientists, and veterinarians, and their scientific and clinical teams, exposes the benefits of xenotransplantation to a broader group and justifies corporate sponsorship beyond those companies engaged directly in xenotransplantation.
The Congress, chaired by Dr. Richard N. Pierson III, touched on a variety of topics of interest to our members. There were many highpoints, including the touching remembrance by friends and colleagues of the late Dr. David White, whom we honored for his life and significant contributions to the field of xenotransplantation. Honorary membership was awarded to Dr. Megan Sykes in recognition of her long time commitment and major contributions to xenotransplantation. Recipients of this year’s TTS-IXA Scientific Awards were announced. We also welcomed new members to the IXA Council. Opening our doors to be even more inclusive and welcoming, sessions were presented for Women in Transplantation and Young Investigators. We look forward to the energy provided by the young investigators, who were well represented in all sessions, as well as that of our new councilors, to keep the IXA moving toward a bright future.

Under the guidance of Dr. Leo Buhler, our journal, Xenotransplantation, has shown a rising impact factor (3.9), highlighting the importance and interest in our work.
We look forward to the 2018 TTS meeting in Madrid and our next IXA meeting, which will be held in Munich on October 10-13, 2019 (see preview). Even in these times of instant virtual communication, the personal interactions and associations developed at the IXA Congress can be very meaningful and rewarding. Please consider joining us there.
These are truly exciting times to be part of the IXA. All those who have an interest in xenotransplantation are encouraged to become active members of the IXA.
Congress Participants:
- Physicians
- Scientists
- Surgeons
- Students

Venue:
University of Maryland, Baltimore
SMC Campus Center
620 West Lexington Street
Baltimore, Maryland 21201 USA
Xeno-Lab
Islet Xenotransplantation Program at the University of Minnesota
Team of Christopher Burlak, Ph.D. at the Schulze Diabetes Institute
The University of Minnesota has established a considerable xenotransplantation initiative focused on developing cellular xenotransplantation as a viable treatment option for patients afflicted with diabetes. To translate basic and preclinical research into clinical islet xenotransplant trials, our program builds on the experience with human islet auto- and allotransplants in nearly 750 recipients and pig-to-monkey islet xenotransplants in more than 100 recipients. Strong institutional commitment has provided an exceptional research infrastructure at the Schulze Diabetes Institute (incl. cGMP facilities for manufacturing genetically engineered cell lines and porcine islet grafts), Preclinical Research Center (GLP transplant studies in the pig-to-nonhuman primate model), Research Animal Resources (biosecure housing of SPF pig donors), and Veterinary Diagnostic Laboratory (state-of-the-art xenodiagnostics). Our program has been substantially strengthened through strategic partnerships with the National Swine Research and Resource Center at the University of Missouri (pig embryology and genetic engineering) and Spring Point Project (designated pathogen-free closed herd of donor pigs). Most importantly, this environment provides my team ample opportunity to interact on a daily basis with a cadre of highly respected investigators (incl. Drs. Bernhard J. Hering, Sab Ramachandran, Melanie Graham, James Collins, Randall Prather, Eric Walters, Tom Spizzo). These investigators contribute complementary expertise in islet transplantation, immunobiology of xenotransplantation, preclinical animal models, xenodiagnostics, genetic engineering, DPF closed herds).

Projects in my group range from genetic engineering of donor pigs to the immunology of xenotransplantation in non-human primates. Our labs at the Schulze Diabetes Institute are located on the beautiful east bank of the Mississippi river. Later this year, a new cGMP facility, dedicated to the manufacture of genetically engineered xenogeneic embryos and cell transplant products will become fully operational. Additionally, newly renovated adjacent laboratory space will accommodate several new postdocs and staff for ongoing projects funded by NIH, JDRF, DRWF, and industry partners.
The genetic engineering of donor pigs has become possible through a long-standing and highly successful collaboration with Drs. Randy Prather and Eric Walters at the National Swine Research and Resource Center. Our teams have generated gene knockout and transgenic pigs and are currently engaged in xenotransplantation studies using mice and nonhuman primates as recipients. The tools for manipulating DNA seem to change every other month and we have taken full advantage of the current technological landscape. Our genetic engineering effort has been focused on creating the minimum number of modifications to a porcine islet donor. After thorough investigation of numerous pig to primate xenotransplantation experiments we focused on reducing NHP antibody binding at the early stages of transplantation through deletion of genes that produce xenoantigens and possibly inclusion of genes that influence the adaptive immune response. Our most recent efforts have produced genetically engineered pigs in just 2 weeks longer than porcine gestation, roughly 4.5 months total. Those pigs are now 1 year old and will become an important part of upcoming experiments.
Working with Dr. Christopher Faulk (Department of Animal Sciences, University of Minnesota) and Dr. Moshe Szyf (Department of Pharmacology and Therapeutics, McGill University) we have designed and optimized a new technique for monitoring islet beta cell destruction after islet transplantation. The work soon to be completed focuses on a highly sensitive technique for measuring the release of durable beta cell specific components into the blood. Through routine blood draws, an islet transplant recipient patient can be monitored monthly for changes in the target molecules for the very earliest signs of rejection. This new assay would allow clinicians to adjust immunosuppressive regimens and also to advance immunologic tolerance of transplanted islets.
We also have two discovery science projects. The first project is a collaboration with Dr. Parastoo Azadi at the Complex Carbohydrate Research Center, where we have analyzed the N-linked and O-linked glycan profiles of human and porcine islets. Our earlier publications have paved the way for a deeper and more thorough investigation into glycan phenotypes among genetically engineered pigs and this new project has provided a clearer picture of what antigens are present as well as the larger scale effect of gene editing on glycosylation enzymes and glycan phenotypes. The second discovery project explores the mRNA expression profiles of samples of porcine islets from highly successful and rejected xenotransplantation experiments. High-coverage RNAseq analysis of islet and pancreas samples has revealed very clear immunologic associations between groups and while this data set is quite new, we are encouraged by the very positive early findings.
We appreciate the opportunity to share with the xenotransplant community a brief description of team, laboratories and some of our projects. We are actively seeking well-rounded postdoctoral fellows in the fields of genetic engineering and informatics and welcome communication from anyone who shares our passion of advancing xenotransplantation into a viable and superior treatment for patients burdened by diabetes.Xeno-Policy
A. The proceedings in clinical trials of corneal xenotransplantation in South Korea
Mee Kum Kim, M.D., Ph.D.1,2,3,4
1 Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea;
2 Laboratory of Ocular Regenerative Medicine and Immunology, Seoul Artificial Eye Center, Seoul National University Hospital Biomedical Research Institute, Seoul, Korea
3 Translational Xenotransplantation Research Center, Seoul National University College of Medicine and Seoul National University Hospital Biomedical Research Institute, Seoul, Republic of Korea
4Executive council, Korean External Eye Disease Society, Seoul, Korea
Corneal blindness is a devastating situation worldwide, and corneal transplantation is the standard treatment for the corneal blindness. The lack of human donors in developing countries demands the exploration of corneal xenotransplantation, and the current nonhuman primate studies using SNU miniature pigs in South Korea suggest that corneal xenotransplantation may be a feasible option in solving the shortage of corneas. Before a clinical trial, we tried to standardized proceedings of the clinical trial in corneal xenotransplantation in South Korea. The protocol has been developed based on an international consensus statement on conditions for undertaking clinical trials of corneal xenotransplantation which had been published by International Xenotransplantation Society in 2014. The protocol has been reviewed by the consulting boards of the executive council of the Korean external eye disease society and the consulting boards of the executive ethical committee of the xenotransplantation research center. Ethical clearance has been sought from the Institutional Review Board of the Ethics Committee of the Seoul National University and the National bioethics committee in South Korea. The protocol of a clinical trial in corneal xenotransplantation using SNU miniature pigs had been submitted on April 14th, 2016, and was approved on April 12th, 2017. This standardization of the proceedings may aid in better and safer clinical research for corneal xenotransplantation in South Korea.
B. Joint FDA-IXA symposium during the IXA Congress, Baltimore, September 20-23, 2017
Following discussions between FDA representatives and the IXA Council, in Boston during the 2016 American Transplant Congress, IXA and FDA organized a joint Symposium during the recent 14th IXA Congress in Baltimore. This half-day symposium, attended by over 250 people, was divided in three sessions:
In the first session, recent scientific advances were reviewed by David Cooper (solid organ xenotransplantation), Berhnard Hering (islet transplantation), and Joseph Tector (genetic engineering)
In the second session, infectious challenges were reviewed by Jay Fishman (lessons from the allotransplant experience), Henk Schuurman (infectious agents and source pigs), Jay Fishman (modified pigs and PERV), and Ralf Tönjes (methods for PERV detection). This session ended with a presentation by David Sachs on the risk-benefit of a xenotransplantation product.
In the third session, regulatory considerations were presented by colleagues from FDA. Judith Arcidiacono gave an introduction and overview including a list of regulatory documents, and this was followed by John Dennis (source animal herds and characterization), Harlan J Howard (regulation of intentionally-altered genomic DNA), Allen Wensky (preclinical considerations), and Bruce Schneider (clinical trials).
All presentations were quite informative and were followed by a lively discussion. Major points raised were the need for pigs expressing regulatory factors of the human complement system and coagulation, in case that new modifications eradicate the possibility of antibody-mediated rejection: this point is relevant but an unequivocal answer is not yet possible. Also the recent publication of live PERV-C-negative piglets in which genes encoding PERV-A and PERV-B are inactivated, raised the question whether such pigs are required for entering clinical trials: this was not considered necessary for first generation xenotransplantation products, but could be considered for a second generation product, if necessary. There was also time to discuss some relevant logistical items, such as transport of designated pathogen-free pigs, housing of multiple genetically modified pig breeds in one facility, and the use of cloned pigs as xenotransplant donors. Also the question was raised whether a multi-transgene product will be considered as one or as multiple drugs in regulatory review. Colleagues from the FDA could not give an unequivocal answer, but mentioned that these aspects will be considered on a case-by-case basis. FDA will accept drugs in Investigational New Drug applications that have not yet received regulatory approval, if proper pharmacology and toxicology data are provided. Also, FDA colleagues mentioned that there is no requirement for a specific species for in vivo testing of a xenotransplantation product, but expect a clear science-based rationale in the selection of a particular species.
In conclusion, this joint symposium was quite successful in presenting the views of the scientific field and the regulatory environment. This topic is relevant today since for a number of xenotransplantation products the entry into clinical trials is on the nearby horizon. IXA members were enthusiastic seeing active interest from representatives from regulatory agencies, not only during the joint symposium, but also at other events during the Congress. This facilitates a good and direct communication, which is needed for the advancement of xenotransplantation to the clinic.
All meeting materials including video recordings of the presentation will be posted on the IXA website: a larger summary and abstracts of presentation will be included in the last issue of Xenotransplantation this year.
Best article in Xenotransplantation Journal

Below is a list of the top 10 downloaded articles in first semester of 2017 in Xenotransplantation.
- Li Y, Wu Q, Wang Y, Li L, Chen F, Shi Y, Bao J, Bu H. “Construction of bioengineered hepatic tissue derived from human umbilical cord mesenchymal stem cells via aggregation culture in porcine decellularized liver scaffolds.”, Xenotransplantation, vol. 24, no. 1, Jan, 2017.
- IXA 2017‐ Abstracts of the 14th Congress of the International Xenotransplantation Association, Baltimore, USA, Xenotransplantation. vol. 24, no. 5, Sep, 2017
- Iwase H, Hara H, Ezzelarab M, Li T, Zhang Z, Gao B, Liu H, Long C, Wang Y, Cassano A, Klein E, Phelps C, Ayares D, Humar A, Wijkstrom M, Cooper DKC, “Immunological and physiological observations in baboons with life‐supporting genetically engineered pig kidney grafts”, Xenotransplantation, vol. 24, no. 2, Mar, 2017
- Gao H, Liu L, Zhao Y, Hara H, Chen P, Xu J, Tang J, Wei L, Li Z, Cooper DKC, Cai Z, Mou L, “Human IL-6, IL-17, IL-1β, and TNF-α differently regulate the expression of pro-inflammatory related genes, tissue factor, and swine leukocyte antigen class I in porcine aortic endothelial cells.”, Xenotransplantation, vol. 24, no. 2, Mar, 2017
- Steffen A, Kiss T, Schmid J, Schubert U, Heinke S, Lehmann S, Bornstein S, Ludwig B, Ludwig S. “Production of high-quality islets from goettingen minipigs: Choice of organ preservation solution, donor pool, and optimal cold ischemia time." Xenotransplantation, vol. 24, no. 1, Jan, 2017.
- Nishimura M, Iizuka N, Fujita Y, Sawamoto O, Matsumoto S. “Effects of encapsulated porcine islets on glucose and C-peptide concentrations in diabetic nude mice 6 months after intraperitoneal transplantation”. Xenotransplantation, vol. 24, no. 4, Jul, 2017.
- Ji H, Long C, Feng C, Shi N, Jiang Y, Zeng G, Li X, Wu J, Lu L, Lu S, Pan D. “Generation of chimeric minipigs by aggregating 4- to 8-cell-stage blastomeres from somatic cell nuclear transfer with the tracing of enhanced green fluorescent protein.”, Xenotransplantation, vol. 24, no. 3, May, 2017.
- Bottino R, Knoll MF, Graeme-Wilson J, Klein EC, Ayares D, Trucco M, Cooper DK., “Safe use of anti-CD154 monoclonal antibody in pig islet xenotransplantation in monkeys.”, Xenotransplantation, vol. 24, no. 1, Jan, 2017.
- Dong X, Hara H, Wang Y, Wang L, Zhang Y, Cooper DK, Dai Y, Pan Z, “Initial study of α1,3-galactosyltransferase gene-knockout/CD46 pig full-thickness corneal xenografts in rhesus monkeys.”, Xenotransplantation, vol. 24, no. 1, Jan, 2017
- Jung SH, Hwang JH, Kim SE, Kim YK, Park HC, Lee HT. “Human galectin-9 on the porcine cells affects the cytotoxic activity of M1-differentiated THP-1 cells through inducing a shift in M2-differentiated THP-1 cells”. Xenotransplantation, vol. 24, no. 4, Jul, 2017
XENO-PRIZE

The 2016 Xeno-Prize was judged recently and we are pleased to announce that the winner is Dr. Lars Burdorf for his paper entitled: “Platelet sequestration and activation during GalTKO.hCD46 pig lung perfusion by human blood is primarily mediated by GPIb, GPIIb/IIIa, and von Willebrand Factor”. Congratulations to Dr. Lars Burdorf and Dr. Andrea Riner (first co-authors) for their outstanding work.
Journal Xenotransplantation New Impact Factor: 3.961
We are proud to announce that the new Impact Factor for our journal is now 3,961, i.e. two points higher than last year. This increase is clearly related to the quality of the submitted papers, i.e. your contributions to the field. We are confident that the development of xenotransplantation and its clinical application will continue to strengthen the interest for the journal. As IXA members, we would ask you to regularly submit articles to the journal and to encourage others to do so. Among the recent innovations, the journal can now be followed on your smartphone by downloading the App “Xen Journal”.
We thank you for your support,
Leo Buhler, MD
Editor in chief, Xenotransplantation
IXA President
Social
Address
International Xenotransplantation Association
C/O The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada




