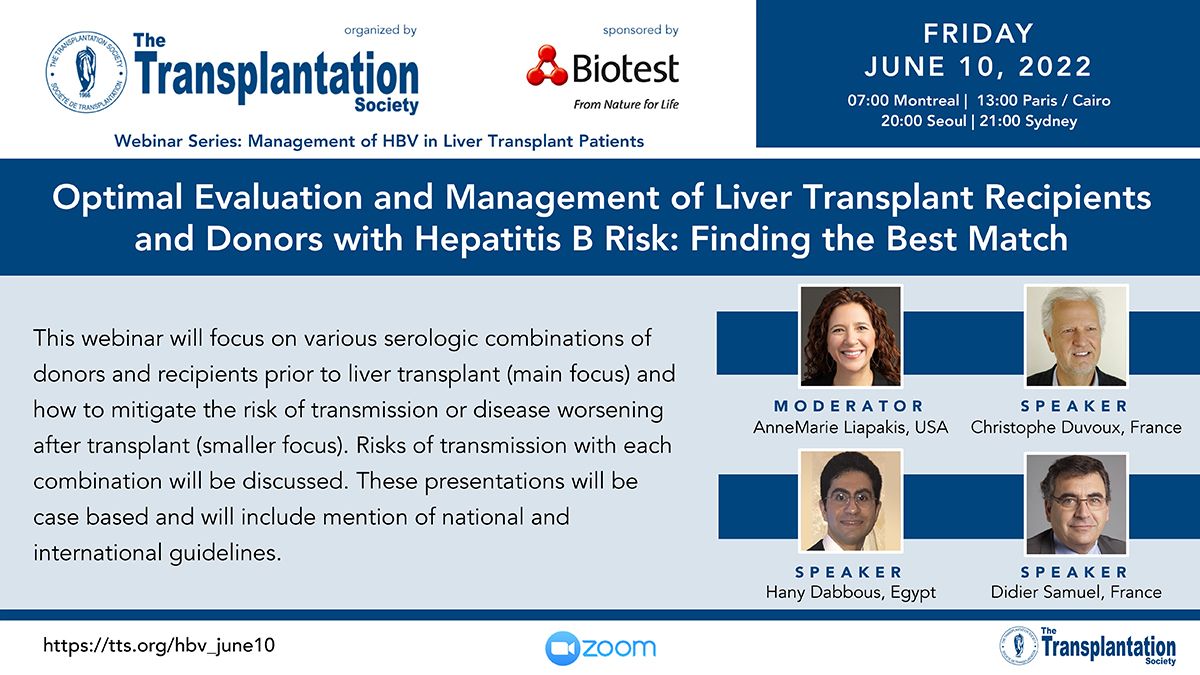
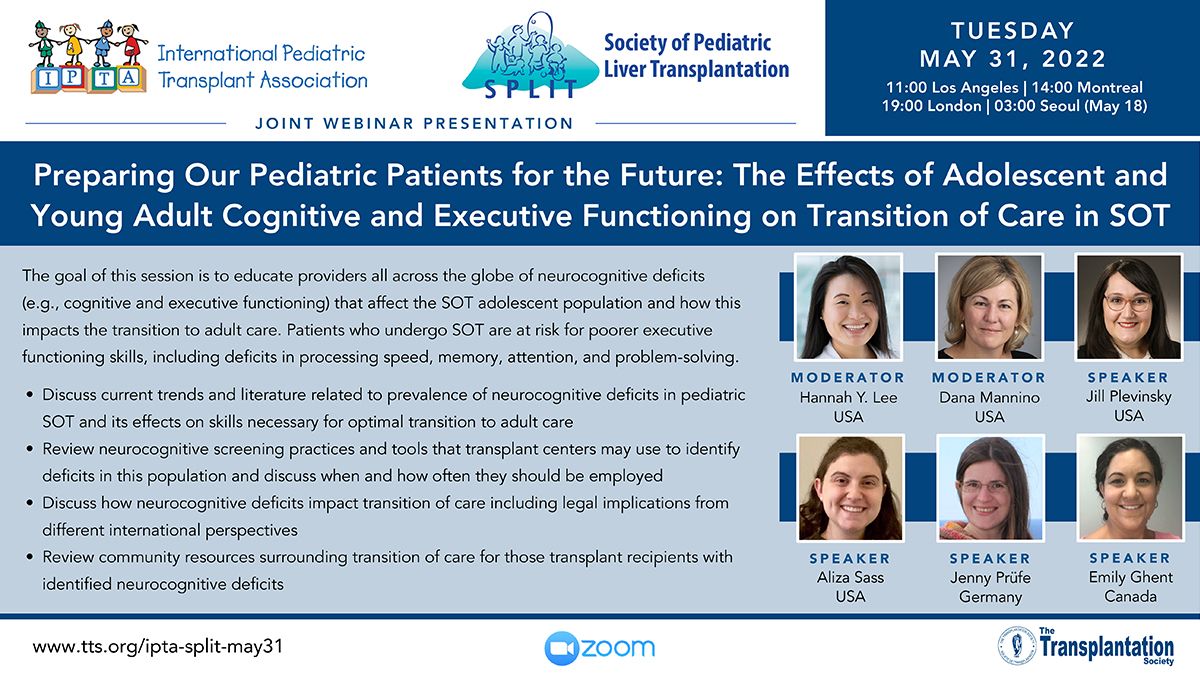
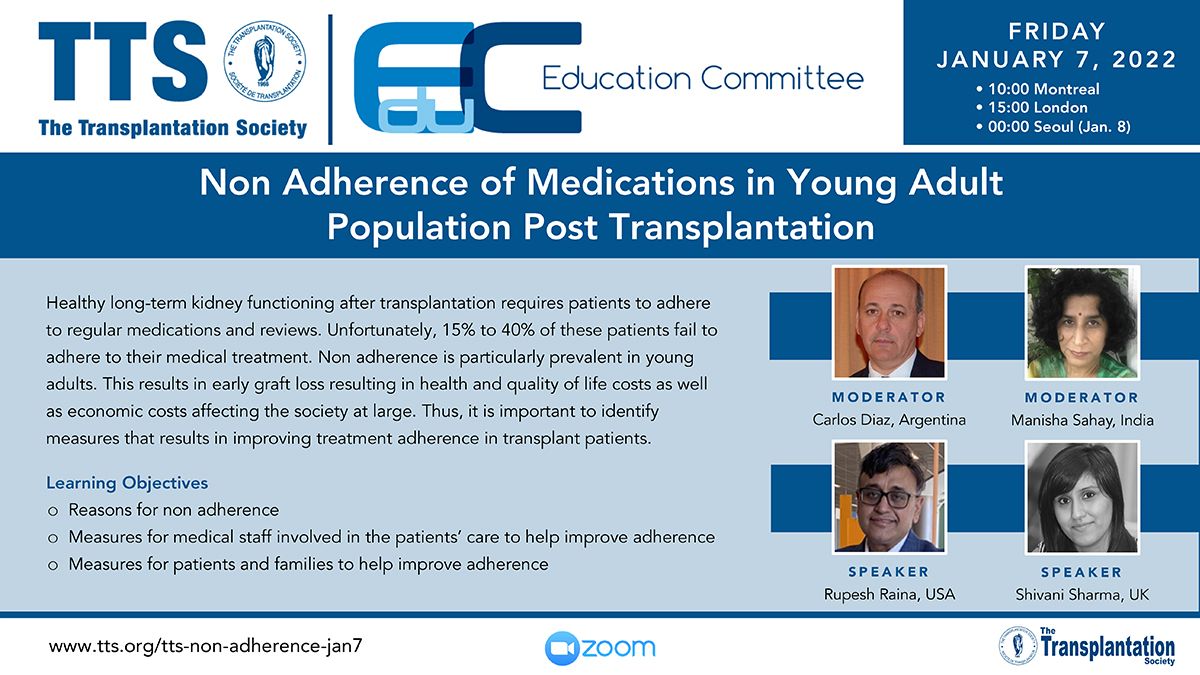
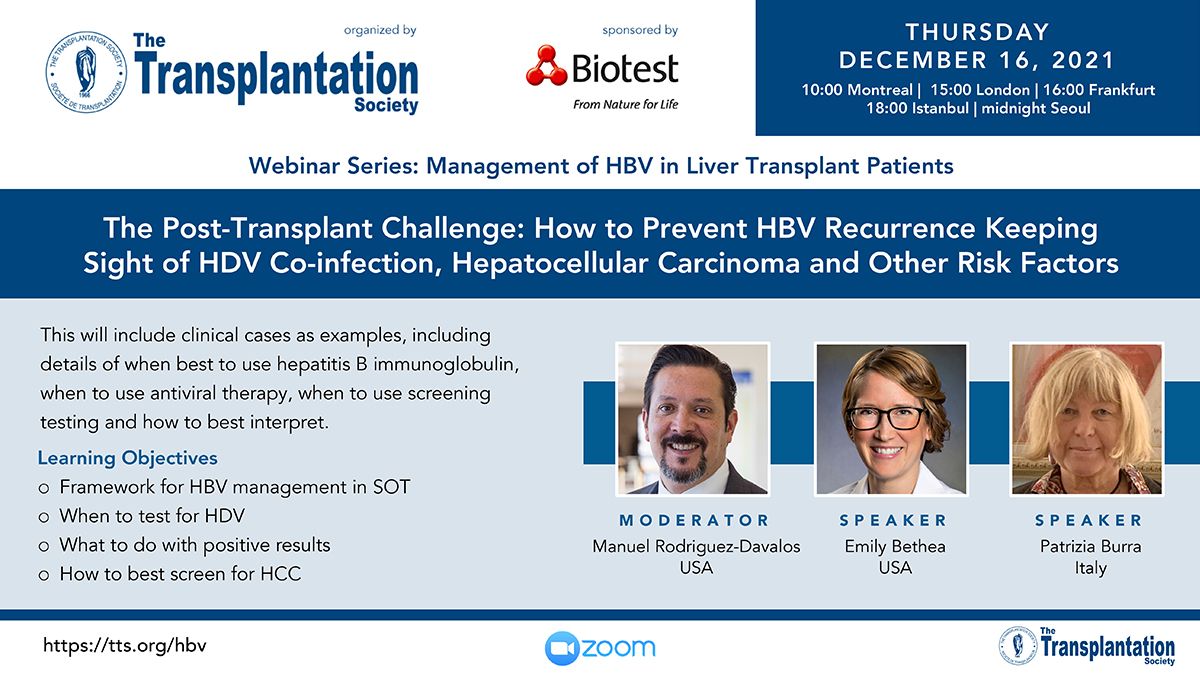
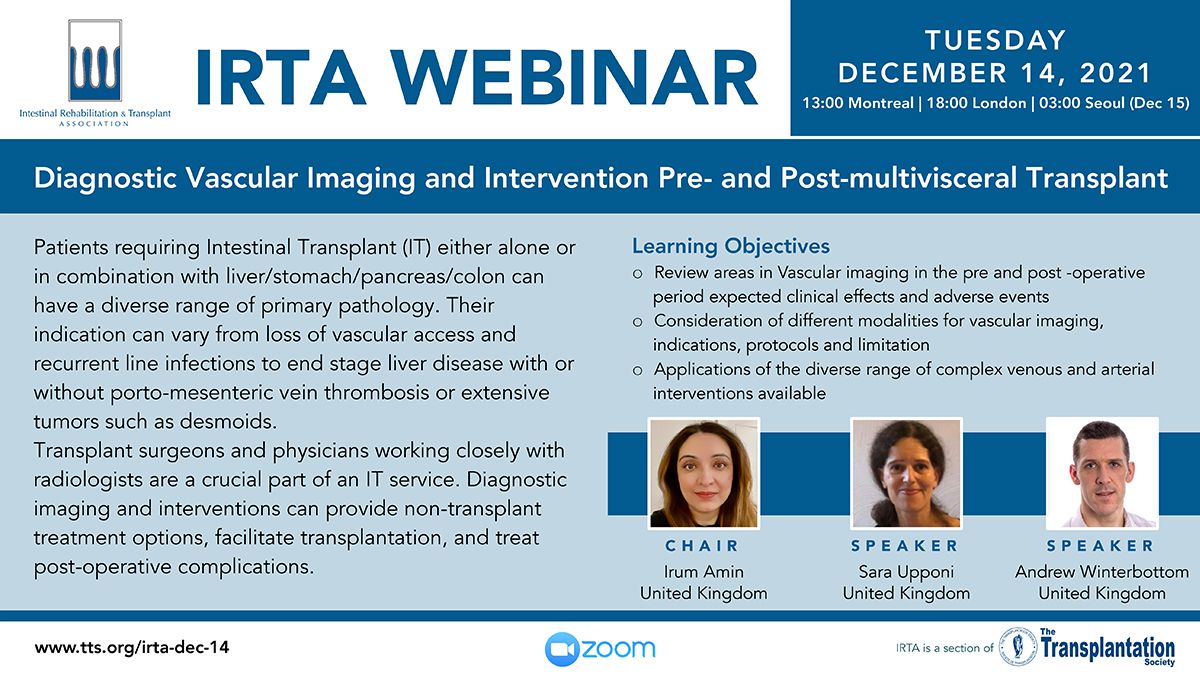
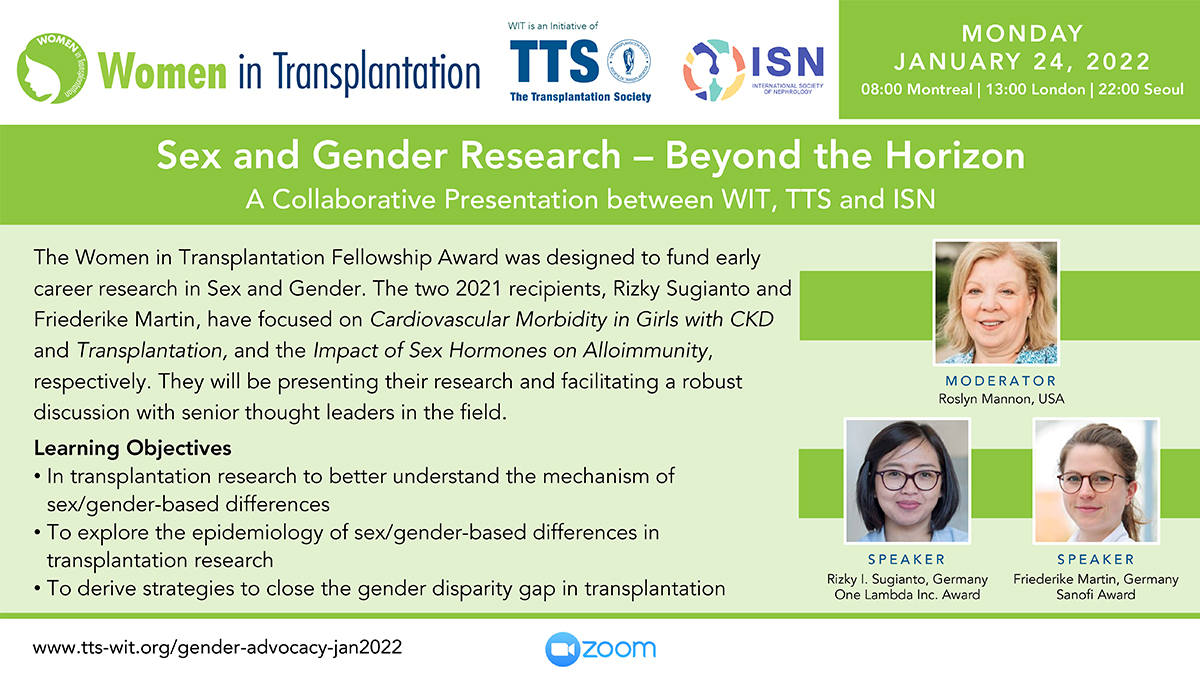
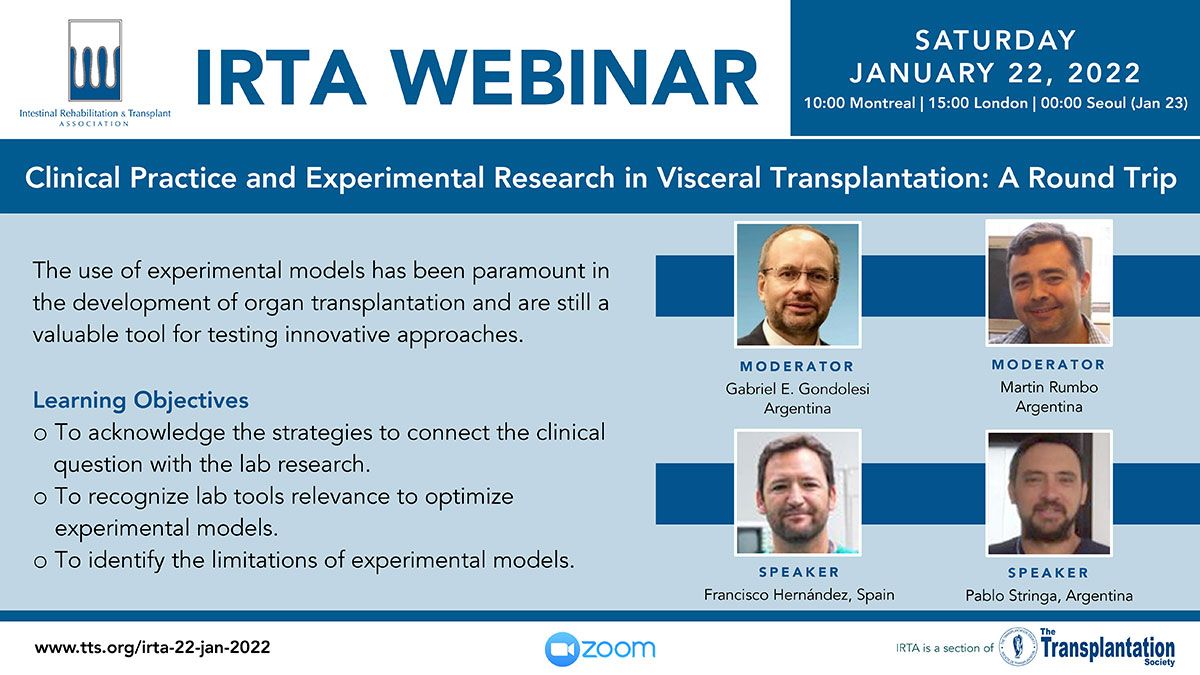
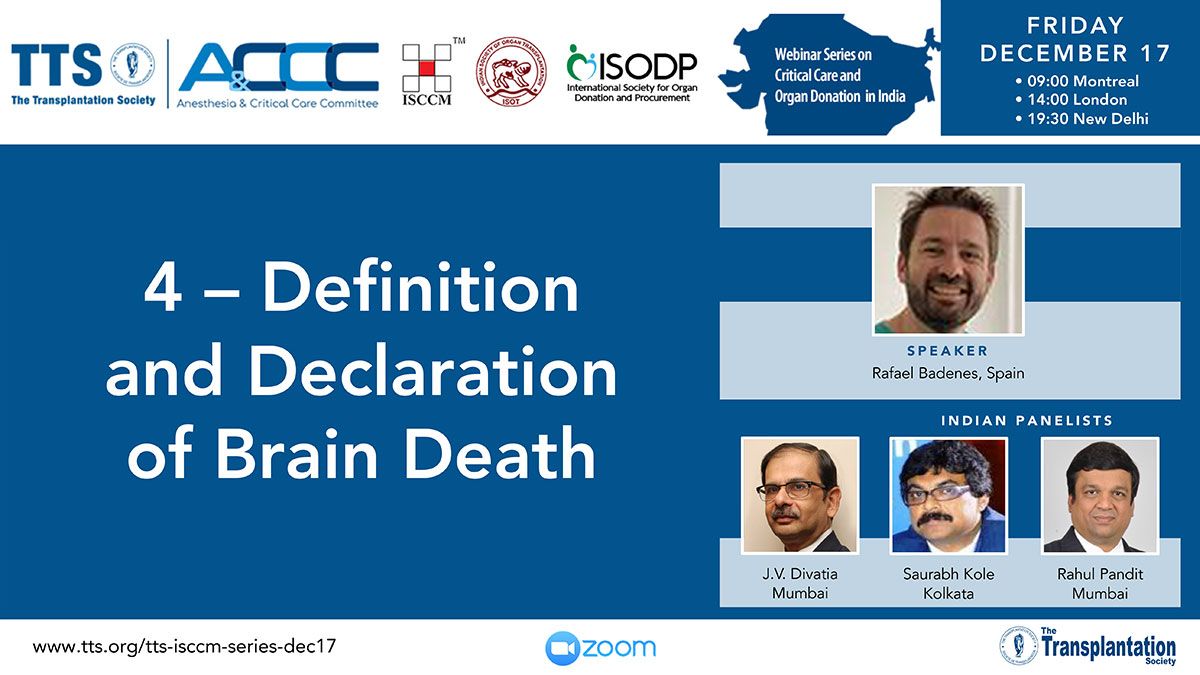
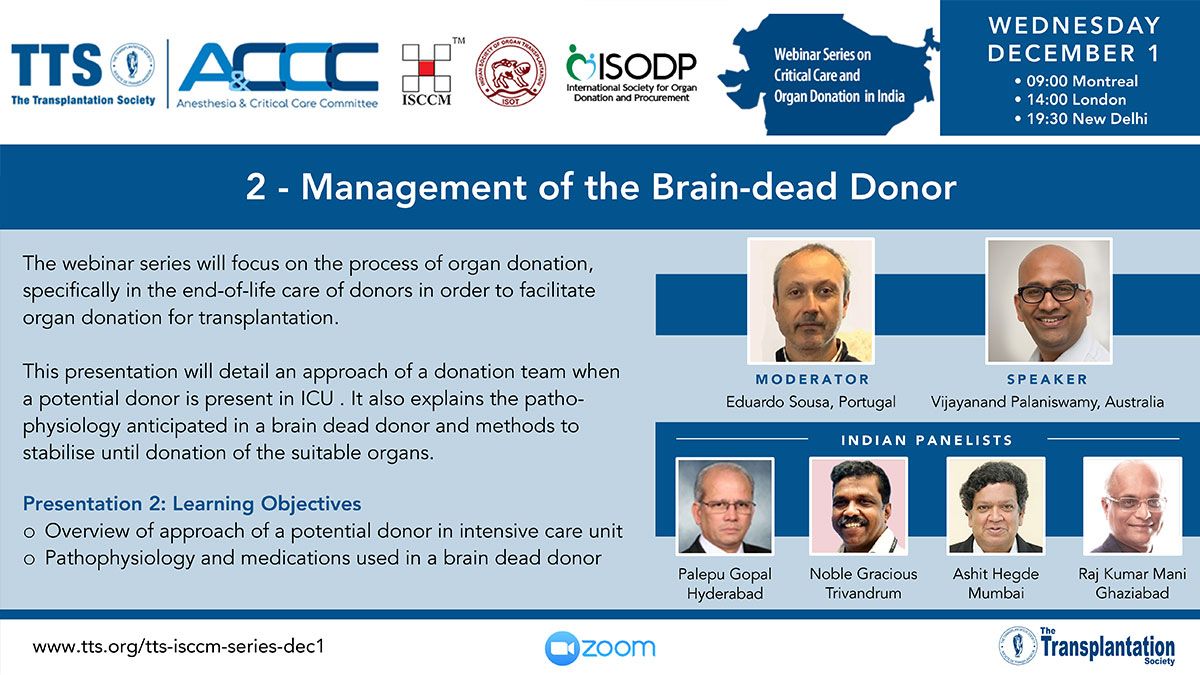
Clinical Decision Support Tool (CDST) for Resistant/Refractory CMV in Transplantation
Cytomegalovirus (CMV) is one of the most common viral infections in the post-transplant setting, including solid organ transplant (SOT) and hematopoietic cell transplant (HCT). CMV conveys a high risk of complications including graft loss, morbidity, and mortality. There are currently no FDA approved therapies for CMV after transplantation and those that are currently in use have serious side effects including kidney and bone marrow toxicity. Data from RMEI medical education programs, as well as information gleaned from CMV experts including TTS Education Committee Member, Camille Kotton MD, show that clinicians who treat CMV in transplant patients need guidance regarding which CMV therapies to choose and how to incorporate a new antiviral (maribavir) in clinical practice.
The Resistant/Refractory Cytomegalovirus Clinical Decision Support Tool (CDST) has been built using the algorithm of the Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation by Camille Kotton, MD et al.
Transplantation Direct
JUST RELEASED - TRANSPLANTATION DIRECT - JULY ISSUE
The July issue of Transplantation Direct is now available online. From the basic sciences, this issue contains articles on the use of an experimental rat model of ischemic lung damage to evaluate potential ex vivo lung perfusion effects. In clinical transplantation, there is a study on the impact of enhanced recovery perioperative care pathway after kidney surgery on early outcomes, and a report on the potential impact of removing race from the kidney donor risk index calculator. In liver transplantation, the question of whether MELD-GRAIL and MELD-GRAIL-Na are better models to predict wait list mortality is investigated; another group explores the use of belatacept to treat persistent TCMR that turns to DSA formation. We also have reports in heart transplantation on combining with liver and kidney transplantation for cases of severe cardiac amyloidosis, and on long term follow-up after heart transplantation for Chagas or non-Chagas cardiomyopathy. Regarding infectious disease, a group investigates CMV infection after COVID vaccination in transplant recipients. Please visit the Transplantation Direct website for full details and open access to these articles.
Basic Science
Experimental Models of Ischemic Lung Damage for the Study of Therapeutic Reconditioning During Ex Vivo Lung Perfusion
Transplantation Direct. 8(7):e1337, July 2022.
Kidney Transplantation
Enhanced Recovery After Surgery Pathway in Kidney Transplantation: The Road Less Traveled
Transplantation Direct. 8(7):e1333, July 2022.
Clinical Utility in Adopting Race-free Kidney Donor Risk Index
Transplantation Direct. 8(7):e1343, July 2022.
Liver Transplantation
MELD-GRAIL and MELD-GRAIL-Na Are Not Superior to MELD or MELD-Na in Predicting Liver Transplant Waiting List Mortality at a Single-center Level
Transplantation Direct. 8(7):e1346, July 2022.
Belatacept Treatment of Recurrent Late-onset T Cell–mediated Rejection/Antibody-mediated Rejection With De Novo Donor-specific Antibodies in a Liver Transplant Patient
Transplantation Direct. 8(7):e1076, July 2022.
Heart Transplantation
Heart Transplantation, Either Alone or Combined With Liver and Kidney, a Viable Treatment Option for Selected Patients With Severe Cardiac Amyloidosis
Transplantation Direct. 8(7):e1323, July 2022.
Long-term Survival Following Heart Transplantation for Chagas Versus Non-Chagas Cardiomyopathy: A Single-center Experience in Northeastern Brazil Over 2 Decades
Transplantation Direct. 8(7):e1349, July 2022.
Infectious Disease
CMV Infection Following mRNA SARS-CoV-2 Vaccination in Solid Organ Transplant Recipients
Transplantation Direct. 8(7):e1344, July 2022.
Transplantation - Week's Most Downloaded Paper
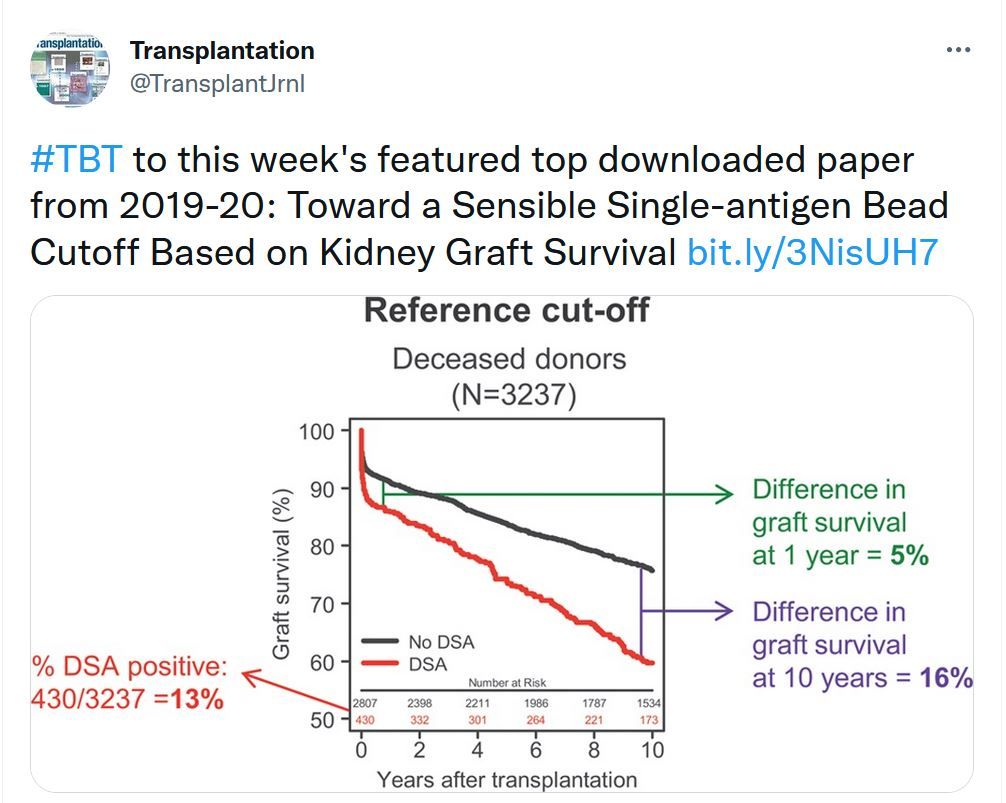
Toward a Sensible Single-antigen Bead Cutoff Based on Kidney Graft Survival #TBT bit.ly/3NisUH7
There is no consensus in the literature on the interpretation of single-antigen bead positive for a specific HLA antibody. To inform the debate, we studied the relationship between various single-antigen bead positivity algorithms and the impact of resulting donor-specific HLA antibody (DSA) positivity on long-term kidney graft survival in 3237 deceased-donor transplants.Transplantation Direct - Week's Most Downloaded Paper
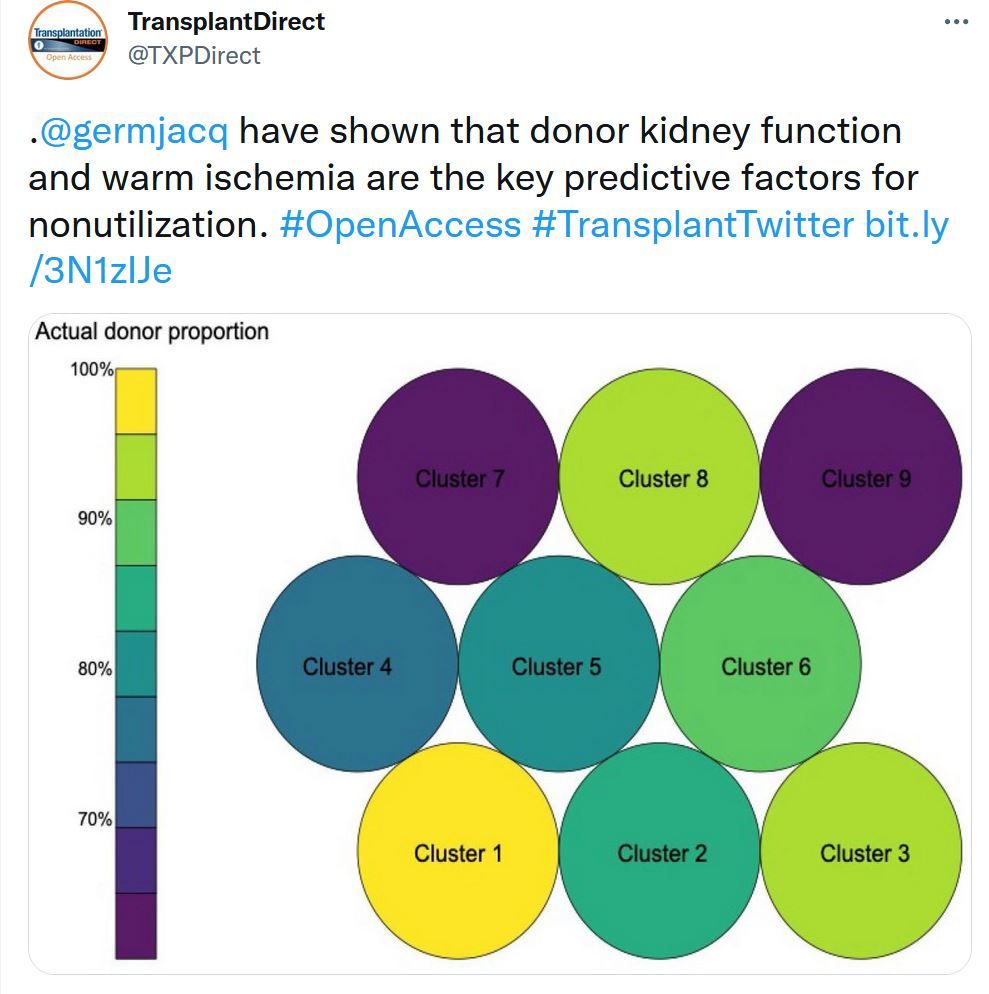
Nonutilization of Kidneys From Donors After Circulatory Determinant of Death #OpenAccess #TransplantTwitter bit.ly/3N1zIJe
The expansion of donation after circulatory determination of death (DCDD) programs and unmet demands for kidney transplantation indicate that there is a need to improve the efficiency and utilization of these organs. We studied all DCDD donors retrieved for kidney transplantation in Australia between 2014 and 2019 and determined the factors associated with nonutilization using least absolute shrinkage and selection operator and random forest models. Self-organizing maps were used to group these donors into clusters with similar characteristics and features associated with nonutilization were defined.TTS Needs Assessment Survey

TTS President
The slogan we adopted for the TTS 2022 Congress is "Committed to Access and Transparency" and as a lead-up to the Congress we have launched a new needs assessment survey which will aid TTS in developing new programs, educational material and strategies to better improve access and transparency in the field of transplantation. As a non-governmental organization in official relations with the World Health Organization (WHO) the information collected will feed into the work TTS is doing as part of our WHO collaboration.
Your support, by completing this survey, will contribute to the success of this initiative. Your answers will be kept confidential and anonymous.
Just Released! IPITA Beta-Cell Bulletin

Recent Recordings and Webinars
June 30, 2022 - Critical Path Institute’s Transplant Therapeutics Consortium (TTC) Webinar
iBox Derived Sample Size Calculator: https://cpath.shinyapps.io/sample_size_ibox_V2/
About the Transplant Therapeutics Consortium (TTC): https://c-path.org/programs/ttc/
Overview: The TTC, in collaboration with leaders from industry, academia, clinical practice, and the US Food and Drug Administration (FDA), hosted this event for stakeholders to discuss pursuing qualification of the iBox Scoring System as a reasonably likely surrogate endpoint with the FDA. The TTC has an accepted Letter of Intent in the FDA Biomarker Qualification Program. In addition, to support the implementation of the iBox Scoring System in future kidney transplant clinical trial design, the TTC developed a graphical user interface for sample size calculation using iBox scores presented in the webinar.
Topics included:
- Pursuing qualification of the iBox Scoring System as a reasonably likely surrogate endpoint
- Clinically meaningful differences in iBox parameters
- Graphical user interface for sample size calculation using iBox scores
Moderator:
- William E. Fitzsimmons, Senior Advisor, Transplant Therapeutics Consortium
Presenters:
- Amanda Klein, Executive Director, Transplant Therapeutics Consortium
- Luke Kosinski, Quantitative Medicine Scientist
Panelists:
- Jiang Liu, Associate Director for Therapeutic Review, Division of Pharmacometrics, Office of Clinical Pharmacology, FDA
- Ulf Meier-Kriesche, Chief Scientific Officer, Veloxis Pharmaceuticals
FDA Letter of Intent: https://www.fda.gov/media/139301/down...
ABOUT C-PATH: Critical Path Institute (C-Path) is an independent, nonprofit organization established in 2005 as a public and private partnership. C-Path’s mission is to catalyze the development of new approaches that advance medical innovation and regulatory science, accelerating the path to a healthier world. An international leader in forming collaborations, C-Path has established numerous global consortia that currently include more than 1,600 scientists from government and regulatory agencies, academia, patient organizations, disease foundations, and dozens of pharmaceutical and biotech companies. C-Path US is headquartered in Tucson, Arizona and C-Path, Ltd. EU is headquartered in Dublin, Ireland, with additional staff in multiple other locations. For more information, visit c-path.org and c-path.eu.
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!